Tetrachloroterephthalonitrile: A Deep Dive
Historical Development
Tetrachloroterephthalonitrile came onto the industrial scene around the mid-twentieth century, pushed by the growing demand within pesticide and specialty chemical markets. Chemists chasing effective agricultural solutions experimented with ring-chlorinated aromatic compounds, and this substance became a standout for its strong performance against fungi and pests. Over the decades, its story reflects a broader trend: the drive toward more targeted synthetic chemicals, particularly as older, less selective treatments fell out of favor due to environmental concerns and mounting regulatory pressure. Crops worldwide owe higher yields, in part, to advances sparked by this molecule, and entire chemical strategies adjusted to accommodate its synthesis and application.
Product Overview
Tetrachloroterephthalonitrile, often found in technical-grade solid form, features most in agricultural fungicides and some specialty polymers. Some chemical suppliers offer it as a near-white to light-gray crystalline powder. Demand may not rival that of glyphosate or atrazine, but it carves out a niche for itself in crop protection formulations where broad-spectrum activity is prioritized. In handling, it calls for well-sealed containers and low-moisture environments to keep its physical form steady and processing issues under control.
Physical & Chemical Properties
This compound stacks up with a molecular weight hovering near 296 grams per mole. Room temperature leaves it in a solid crystalline state, with a melting point sitting around 220–230°C, a mark that demands some respect during manufacturing and storage. Tetrachloroterephthalonitrile dissolves poorly in water but shows better results in solvents like acetone or chloroform—practical knowledge for anyone running reactions or formulating products. Its chlorinated aromatic ring gives it durability in harsh conditions, while the dinitrile groups provide many paths for downstream chemical modification. Chlorine atoms built into its structure don’t just resist degradation; they invite attention from anyone looking to build tracers or specialty polymers designed for tough settings.
Technical Specifications & Labeling
Suppliers usually guarantee at least 98% purity, often providing batch-specific gas chromatography analysis and impurity profiles. Technical sheets list melting point, solubility, and storage instructions, alongside required hazard pictograms. The CAS number, 117-18-0, sits alongside recommended handling procedures: keep workspaces well-ventilated, avoid exposure to open flames, and equip staff with gloves and respirators. Labeling also warns about environmental persistence, reflecting current thinking on responsible chemical stewardship.
Preparation Method
Industrial chemists turn to a multi-step route, usually starting with tetrachlorobenzoic acid, transforming it with a dehydration or amide condensation process in the presence of strong dehydrating agents like phosphorus oxychloride or thionyl chloride. The process runs under controlled temperature and often under reflux within a closed reactor to contain volatiles. Purification collects the material through sequential filtration, washing, and recrystallization—routines that chemical engineers fine-tune to balance purity, yield, and waste. Running these steps at larger scales, shops pay close attention to managing toxic byproducts and minimizing off-gassing.
Chemical Reactions & Modifications
Because of its four chlorine atoms and paired nitrile groups, tetrachloroterephthalonitrile acts as a flexible building block for synthesizing specialty chemicals. Some research groups use nucleophilic aromatic substitution, where chlorine atoms swap out for amine or thiol groups, unlocking routes to complex ligands or new plastics for electronics. The nitrile groups prove useful when chemists want to build longer chains, or convert them into amides, acids, or amidoximes with straightforward reagents. These modifications open doors for custom fungicides, dyes, or material additives, each spinning off from the central rigid aromatic core.
Synonyms & Product Names
Tetrachloroterephthalonitrile pops up under several names, making it a challenge to track unless the context is clear. Industry veterans call it TC-TN, and its longer names include 2,3,5,6-tetrachlorobenzene-1,4-dicarbonitrile or the trade name chlorothalonil in certain applications. Documentation often toggles between these choices depending on application—regulatory filings stick to IUPAC or CAS identifiers, while market-facing material uses brand names that can overlap with specific formulations.
Safety & Operational Standards
Any shop or farm handling tetrachloroterephthalonitrile keeps close watch over safety. Workers suit up in gloves, goggles, and filtered masks; facilities run local extraction fans at points of dust or fume release. Documented toxicity means spills demand rapid cleanup, and any waste moves in sealed drums to approved incinerators. Regulatory bodies recommend frequent training—understanding not just emergency procedures but the subtle drag of chronic exposure. Every year brings a new round of inspections and record-keeping, and producers live with the knowledge that lapses in protocol can mean serious harm. In research labs, smaller quantities still prompt vigilance—fume hoods stay busy, and risk assessments go beyond paperwork.
Application Area
Large-acre farming and turf management turn to tetrachloroterephthalonitrile for its ability to block mold, rot, and blight across fruits, vegetables, and grains. Golf courses and sports fields also get treatments, thanks to both broad-spectrum action and the way this chemical persists on plant surfaces after rain. For industrial teams focused on materials, the compound acts as a precursor in specialty polymer and resin synthesis; engineers value its stability and chlorine content where flame retardance is prized. Smaller pockets of the market include pigment producers and some textile treatments, each drawing on unique features of the chemical structure to deliver function.
Research & Development
Academic and corporate labs continue to study new ways to make and use tetrachloroterephthalonitrile. Teams keep looking for routes that create less waste, use safer reagents, or enable greener purification methods—shrinking water and solvent priorities down step by step. Some researchers push boundaries by trying catalysts that knock out side reactions, or one-pot syntheses that save energy and time. On the downstream side, biochemists explore modified versions to solve shifting pest resistances and patch up the performance lost to older, phased-out compounds. Partnerships between universities and manufacturers mean the pace of experimentation stays high, as pressure rises from customers and regulators both.
Toxicity Research
Toxicologists trace acute and chronic effects of tetrachloroterephthalonitrile on animals and humans with growing detail. Acute exposure risks focus on skin and eye irritation, but long-term studies signal deeper trouble such as carcinogenic effects, liver and kidney stress, and impacts on aquatic life. The substance falls on regulatory watchlists across several continents; groundwater contamination and worker exposure drive most of the studies. Environmental persistence—one of the molecule’s selling points for crops—becomes a problem downstream. Laboratories keep refining animal models and cellular assays to probe mechanisms of toxicity, while public health outfits campaign for tighter limits, better testing, and follow-up on people living near application zones.
Future Prospects
Companies and scientists working with tetrachloroterephthalonitrile face twin pressures: safeguard yield and cut risk. Many solutions begin on the formulation bench, where dosing, packaging, and delivery tricks aim to reduce loose powder and drift. Some research targets replacement altogether, hunting for molecules with similar protection but better environmental profiles. Biotech advances make it possible to engineer crops or microbes for innate resistance, knocking down the need for heavy-duty outside intervention. Regulatory agencies call for transparency—every step from synthesis to field application and residue testing stands open for scrutiny. For those who grew up seeing this chemical as an everyday tool, the landscape has changed: now, innovation means stretching benefits while respecting tight new boundaries and the health of both workers and the world around them.
A Chemical With Surprising Reach
Tetrachloroterephthalonitrile doesn’t roll off the tongue, and it rarely grabs headlines. Yet, its presence reaches from farm fields to shelves in almost every hardware store. Known in manufacturing and industry circles for decades, this compound has left its mark thanks to its effectiveness against stubborn weeds and its utility in other chemical processes.
From Farmland to Factory
For most people, the mention of Tetrachloroterephthalonitrile immediately brings up one use: making the herbicide chlorothalonil. Farmers face weeds and fungi every year, and losing crops isn’t just an inconvenience. It means shrinking profits, less food, and wasted resources. When chlorothalonil landed in the market back in the 1960s, it changed the game for producers of peanuts, potatoes, tomatoes, turfgrass, and more.
My own family’s small commercial greenhouse wouldn’t have survived some outbreaks without it. Fungi wipe out seedlings faster than most folks expect, and fungicides containing derivatives of Tetrachloroterephthalonitrile helped keep the harvest coming. Data from the U.S. Environmental Protection Agency show chlorothalonil consistently ranks among the top fungicides nationwide, especially for vegetables that rot easily in damp climates.
Beyond Agriculture
It’s tempting to pin Tetrachloroterephthalonitrile’s utility on farming alone, but there’s a bigger story. Production plants rely on this compound as a building block for dyes, resins, and other specialty chemicals. That blue pool liner you see at the public pool? The pigment probably owes something to this molecule. Several textile dyes still use chemical processes where intermediates come straight from Tetrachloroterephthalonitrile. Here technology and chemistry team up; advances in dye chemistry allow brighter, longer-lasting colors, and this compound plays a supporting role.
Paint manufacturers lean on products derived from Tetrachloroterephthalonitrile, not just for color but for resilience against mildew and UV rays. Outdoor surfaces like decks, siding, and fences take a beating, and mildew loves damp, shady spots. Using chemicals with a proven track record helps companies back up durability claims.
Safety and Sustainability Concerns
Plenty of people ask questions about safety. Synthetic chemicals always raise flags for environmentalists and public health watchdogs. The EPA has flagged chlorothalonil contamination in some waterways, raising concern about runoff from treated fields. Studies link the chemical’s persistence in soil and water to risks for fish and aquatic insects.
I remember local debates when the town’s creek showed traces of agricultural chemicals. Neighbors, especially those with kids and pets, wanted answers about how much exposure counts as dangerous. The World Health Organization has listed chlorothalonil as a probable carcinogen, making calls for tighter controls louder than ever.
Working Toward Safer Solutions
Changing the way farmers and manufacturers use Tetrachloroterephthalonitrile means considering both the benefits and the drawbacks. Practices like targeted application, buffer zones along waterways, and alternative pest controls help reduce unnecessary runoff. Research doesn’t stop either. University and private lab scientists work on replacements that tackle mold, weeds, and mildew without the long-term environmental costs.
Where we go from here depends on honest conversations between producers, government agencies, and people living near farms and factories. For now, Tetrachloroterephthalonitrile keeps playing a behind-the-scenes role, making food supplies more stable and products more resilient, while society figures out how to balance progress and stewardship.
What Tetrachloroterephthalonitrile Is and Why Handling Matters
Tetrachloroterephthalonitrile, known by the trade name Dacthal, often pops up in conversations about pesticides. I’ve seen it listed on labels in storage closets and forgotten behind old shelves in agricultural supply stores. This substance doesn’t behave like the flour and sugar you keep in the kitchen—far from it. Dacthal’s dust and crystals can quickly become a health risk. It’s got a track record for causing eye, skin, and respiratory irritation, and breathing in the stuff may do long-term harm. Mistakes in storage, spills, or even casual handling could bring real trouble.
Personal Experience: The Basics Matter Most
I once worked in a greenhouse that kept stricter chemical controls than most hospital emergency rooms. The folks who skipped gloves or goggles always paid the price with rashes or coughing fits. These aren’t just safety fairy tales—they’re the regular reality when dealing with harsh chemicals like Tetrachloroterephthalonitrile. Experience hammered home the idea that cutting corners never pays off. Simple steps add up to real protection.
Dress for the Job—Don’t Take Shortcuts
People might shrug off goggles or dust masks, but splashy labels warn about eye burns and skin problems for good reason. Dacthal can sting, burn, or leave lasting patches. Always pull on chemical-resistant gloves. Rubber or nitrile work best. Protect your eyes with wraparound goggles and shield your skin with a long-sleeve shirt and solid, non-absorbent pants. Breathable dust masks, certified particulate respirators, or even a full-face shield keep lungs safe when dust clouds form. Don’t trust a bandana—use real, tested gear. I once saw a friend think a scarf would do the trick, only to end up wheezing and red-eyed.
Keep It Away from Food, Water, and Everyday Spaces
Chemicals and snacks don’t mix. Store Dacthal and anything like it far from water fountains, lunch tables, and public walkways. Lock supplies in a dry, well-ventilated spot that stays cool and never gets sunlight. Good storage keeps toxic dust from drifting silently onto hands, utensils, or shoes headed back home. Label everything clearly. Mixing up pesticides in a rush has caused more than one serious accident.
Clean-Up and Spills: Fast, Not Careless
Accidents don’t wait for a free afternoon. If Dacthal spills, act quickly—shovel crystals into a strong, sealed bag or drum before sweeping the last specks. My old boss kept big bins of activated charcoal on hand for soaking up liquid chemical spills. Ventilate the area, keep bystanders out, and wipe surfaces with disposable towels. No spray bottles of air freshener or clever hacks—just old-fashioned muscle and reliable supplies. Any contaminated clothes need to go right in the laundry, separate from the rest.
Training, Trust, and Teamwork Beat Overconfidence
Story after story from factories, farms, and workshops always circles back to the same truth: people who know the risks get hurt less often. Ongoing training, honest supervision, and tight-knit teams keep mistakes small. Recklessness or pride causes more pain than the chemical itself. Reporting symptoms early, reading up on emergency eye wash or shower stations, and learning antidotes or first aid could save a life.
Solutions: Making Safety Second Nature
Prevention beats reaction. Update training every season. Bring in professionals whenever you change products or see a process you don’t understand. Build a shared sense of watchfulness, so no one gets left alone in the backroom with unsafe habits. A culture that puts safety first doesn’t grow out of paperwork—it begins with real conversations and respect for both experience and expertise.
What Is Tetrachloroterephthalonitrile?
Tetrachloroterephthalonitrile, sometimes called Dacthal or DCPA, shows up mostly as a herbicide in agriculture. Farmers have used it for decades to control weeds in crops and lawns. Products containing this compound get spread on fields and can end up in the air, soil, and water. People in neighboring communities can encounter it, especially near treated farmland, parks, and golf courses.
Health Concerns
I live near a farming region and remember community meetings where residents voiced worries after reading pesticide labels. They wondered what happens if Dacthal drifts into the air or runoff enters drinking water. Studies have flagged some health risks. According to the U.S. Environmental Protection Agency, DCPA breaks down into chemicals that can contaminate groundwater. These byproducts can hang around in the environment for months. Some studies in animals point to impacts on the liver, thyroid, and the developing nervous system after long-term exposure. Even though government agencies set limits and rules for safe use, the possibility of inhaling the dust or getting it in well water puts folks on edge.
Scientists have not linked short-term, everyday exposures to immediate major health problems, but the deeper concern focuses on chronic low-level contact. People with jobs spraying this herbicide carry a bigger risk because of repeated handling and the chance for skin contact or breathing contaminated dust. Kids face extra danger if they play on treated grass soon after application since their bodies are still growing and more sensitive to chemicals.
Environmental Impact
Treated fields often stretch alongside streams and wildlife habitats. After heavy rain, runoff can push DCPA and similar chemicals into waterways, causing stress for fish and water insects. Reports from the US Geological Survey flag the presence of DCPA in surface water and some groundwater samples. Over time, these residues can travel far past their original fields, affecting wetland ecosystems and nearby drinking water wells.
Wildlife pays the price. Amphibians, for example, have thin skin that absorbs chemicals straight from the water. Repeated chemical exposure disrupts their development and reproductive cycles. Direct spraying harms beneficial insects like bees and butterflies, pushing their numbers lower every year.
Finding Safer Paths Forward
Safer alternatives do exist. Many farming communities now experiment with cover crops, crop rotation, or mechanical weed removal. While these methods sometimes take more labor or adjustment from longtime routines, they cut the need for chemicals like DCPA. People living near farms can advocate for steps like planting buffer strips of native grasses alongside waterways or posting clear signs when herbicides get applied, so families stay informed and out of danger zones.
Water monitoring provides an early warning. I remember local agencies sharing test results with the public and offering home water filters or supporting well testing for residents worried about contamination. Open dialogue between farmers, scientists, and neighbors often leads to creative problem-solving. Keeping an eye on new research, stricter rules, and technology helps make sure that convenience or short-term cost savings never outweigh the health and safety of people, animals, or the environment.
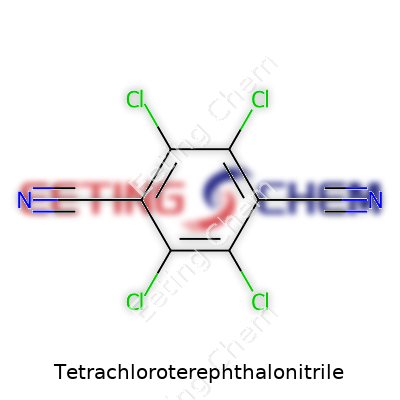
Unpacking the Formula: C8Cl4N2
Chemistry can sound like a maze, but some compounds play a quietly powerful role in our lives. One such material, Tetrachloroterephthalonitrile, delivers both in complexity and value. Its formula, C8Cl4N2, reveals quite a bit. That shorthand means it contains eight carbon atoms, four chlorine atoms, and two nitrogen atoms. If you map out the atoms, you see a benzene ring at the core—something found across countless industrial chemicals—with four chlorine atoms and two nitrile groups attached. The arrangement isn’t just for show; it changes the compound’s behavior in dramatic ways.
Breaking Down the Structure
Imagine the classic six-sided ring of benzene. In this case, the structure holds chlorine atoms at the 2, 3, 5, and 6 positions. At opposite ends of the ring, you find the two nitrile groups, sitting at the 1 and 4 spots. The presence of nitrile groups groups gives the molecule unique capabilities. Nitrogen doesn’t play by the same rules as carbon or chlorine, so you get a compound that’s both chemically stable and reactive in precisely targeted ways. Having worked with specialty chemicals in industrial labs, I can say that subtle structural tweaks like these set the great apart from the mediocre in polymer chemistry or agrichemicals.
What Makes Tetrachloroterephthalonitrile Important
Factories and research plants embrace molecules like this because they enable the synthesis of other complex compounds, dyes, and advanced polymers. Resins reinforced with such additives can offer greater resistance against weathering, UV light, and many types of chemical corrosion. With the chlorine atoms locking into those specific spots on the ring, the material resists being broken down by water or heat. Engineers and scientists find themselves reaching for this compound when they need a tough-yet-versatile intermediate.
Real-world use stretches from protective coatings to specialty materials found in electronics and aerospace. The unique blend of stability and reactivity gives manufacturers options in fine-tuning their final products. From personal experience, these properties mean designers have a bigger toolbox for innovation in tough environments, from circuit boards to outdoor infrastructure.
Health and Environmental Responsibility
With all synthetic chemicals, the story rarely ends at performance. Four chlorine atoms draw attention from health and environmental specialists worldwide, since chlorinated aromatics have a tight connection to toxicity or bioaccumulation. Strict handling and disposal procedures help manage risk. In labs I’ve worked, protective gear and proper air circulation always ruled the day whenever working with halogenated organics, for good reason.
Clear rules and careful monitoring cut down on waste leaks or improper exposure. Chemists and engineers keep watch for safer alternatives with similar performance, especially as regulations tighten in many countries. Some efforts focus on tweaking the formula or swapping out chlorine for less troublesome atoms. That pursuit hinges on balancing safety, reliability, and cost—an ongoing challenge across the chemical industry.
Moving Forward with Knowledge
Tetrachloroterephthalonitrile’s example teaches something bigger about chemistry in daily life. Know what goes into these processes and you start to see choices that shape health, technology, and sustainability. Every atom’s place—and the bonds they share—changes what’s possible. Staying curious about the “why” behind such compounds opens up better solutions for the next round of questions society faces.
Storing Tetrachloroterephthalonitrile: Everyday Prudence with Heavy Chemicals
Tetrachloroterephthalonitrile isn’t a chemical I’d want to ignore in the back of any warehouse. This substance shows up in specialty manufacturing, sometimes with little fanfare, but the wrong move with it can cause trouble. Bleached white crystals might look harmless, but they can turn volatile, especially if left where heat and moisture sneak in.
At a job I once had in industrial coatings, big drums of organic chemicals stood in cages with fixed vents overhead. A guy new to the team left a barrel near a leaking roof one summer, and it didn’t take long for warning alarms to start. Moisture attacks a lot of chemicals—Tetrachloroterephthalonitrile fits that category—leading to slow breakdown or toxic gas buildup. Dry, cool conditions make a huge difference, and no one should underestimate a secure, climate-controlled storage room.
False thrift sometimes leads plant managers into trying to “store just for a short while” in less-than-ideal sheds or near open windows. Putting Tetrachloroterephthalonitrile on a high metal shelf above powerful solvents gives the worst of both worlds, since cross-contamination and corrosion speed up risk. Evidence from multiple OSHA incident reports always highlights poorly ventilated or makeshift storage as an early cause of chemical issues.
It just takes one cracked label or loose cap for dust or vapor to escape. Regular inventory checks, along with tight, chemical-resistant containers, help companies avoid headaches down the line. Locking access, signage that calls out the hazard, and training sessions all add layers of practical protection.
Disposal: Cutting Corners Wrecks More Than the Bottom Line
There’s sometimes a temptation to call hazardous waste disposal “just another expense,” but the risks tend to boomerang. If Tetrachloroterephthalonitrile gets poured down the drain, the damage can show up both inside a factory and in the wider community’s water supply. Years ago, I saw the fallout when a warehouse tried to treat complex wastes like kitchen trash—a cleanup that choked an entire industrial park for weeks.
EPA documentation points toward incineration at high temperatures in regulated facilities as the preferred route. Burning at low heat might release unreacted cyanide or dioxin-type byproducts, so certified waste handlers bring specialized equipment. Professional disposal companies use manifests that explain exactly what goes in the truck, giving both peace of mind and a paper trail. This kind of careful record-keeping proves invaluable if a regulatory audit or spill puts a business under scrutiny.
Landfills never count as a shortcut. Burying toxic material sets the stage for long-term environmental damage, sometimes surfacing decades later as leached pollution. I’ve met small business owners who later learned that old landfill habits left their brand tangled with lawsuits and cleanup fees.
Practical Steps Forward
Staff training can’t just be a slide show. Walking through real storage spaces, checking weatherproof seals, and running drills on proper spill response drills all anchor safety habits. For disposal, working with certified handlers reduces liability and keeps neighborhoods out of danger. Investing in good labeling, updated chemical inventories, and locked cabinets pays off with fewer emergencies and insurance issues.
Tetrachloroterephthalonitrile doesn’t belong on the long list of chemicals no one thinks about until something explodes or leaks. Staying sharp with safe practice turns a routine task into peace of mind—on the job and in the community.
| Names | |
| Preferred IUPAC name | 4,5,6,7-Tetrachlorobenzene-1,2-dicarbonitrile |
| Other names |
Cyanolime
C8Cl4N2 Tetrachloro-1,4-benzenedicarbonitrile TCNB Chlorothalonil |
| Pronunciation | /ˌtɛ.trəˌklɔːr.oʊ.tɛˌrɛf.θəˈloʊ.nəˌtraɪl/ |
| Preferred IUPAC name | 1,2,4,5-Tetrachlorobenzene-1,4-dicarbonitrile |
| Other names |
Cyananil
Chloranil Cyananil T TCTN Tetrachloro-1,4-benzenedicarbonitrile |
| Pronunciation | /ˌtɛtrəˌklɔːrəˌtɛrəfˌθæləˈnaɪtraɪl/ |
| Identifiers | |
| CAS Number | 117-08-8 |
| Beilstein Reference | 1636059 |
| ChEBI | CHEBI:34718 |
| ChEMBL | CHEMBL3720896 |
| ChemSpider | 24509 |
| DrugBank | DB14015 |
| ECHA InfoCard | 100.028.778 |
| EC Number | 206-116-6 |
| Gmelin Reference | 82167 |
| KEGG | C18594 |
| MeSH | D013755 |
| PubChem CID | 8577 |
| RTECS number | SM8575000 |
| UNII | NLV8X2UJ2N |
| UN number | UN3347 |
| CompTox Dashboard (EPA) | DTXCID30408 |
| CAS Number | 117-18-0 |
| Beilstein Reference | 1722082 |
| ChEBI | CHEBI:34918 |
| ChEMBL | CHEMBL514661 |
| ChemSpider | 21172261 |
| DrugBank | DB08604 |
| ECHA InfoCard | 100.011.699 |
| EC Number | 204-355-4 |
| Gmelin Reference | 199066 |
| KEGG | C19780 |
| MeSH | D013749 |
| PubChem CID | 8551 |
| RTECS number | SK1050000 |
| UNII | 8WI54YWN1Q |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DJ198S278O |
| Properties | |
| Chemical formula | C8Cl4N2 |
| Molar mass | 317.93 g/mol |
| Appearance | White to light tan crystalline solid |
| Odor | Odorless |
| Density | 1.8 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.94 |
| Vapor pressure | 1.59E-7 mmHg |
| Acidity (pKa) | 1.2 |
| Basicity (pKb) | pKb: 3.77 |
| Magnetic susceptibility (χ) | -0.000123 |
| Refractive index (nD) | 1.613 |
| Viscosity | 1.21 cP (25°C) |
| Dipole moment | 0.0 D |
| Chemical formula | C8Cl4N2 |
| Molar mass | 291.93 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.8 g/cm³ |
| Solubility in water | insoluble |
| log P | 3.9 |
| Vapor pressure | 2.84 x 10^-7 mmHg (25°C) |
| Acidity (pKa) | Acidity (pKa): -0.41 |
| Basicity (pKb) | 13.7 |
| Magnetic susceptibility (χ) | -0.000141 |
| Refractive index (nD) | 1.6440 |
| Viscosity | 1.39 cP (25°C) |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 290.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −239 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1281 kJ mol⁻¹ |
| Std molar entropy (S⦵298) | 256.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -83.1 kJ mol⁻¹ |
| Hazards | |
| Main hazards | Suspected of causing cancer. Very toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H315, H317, H319, H335, H400 |
| Precautionary statements | P261, P264, P270, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0-🛢️ |
| Autoignition temperature | 690 °C (1274 °F; 963 K) |
| Lethal dose or concentration | LD50 oral rat 1700 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 7400 mg/kg |
| NIOSH | TTZ000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.11 mg/m3 |
| IDLH (Immediate danger) | 20 mg/m3 |
| Main hazards | Harmful if inhaled, causes skin and eye irritation, may cause allergic skin reaction, very toxic to aquatic life. |
| GHS labelling | GHS02, GHS07, GHS08, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H315, H317, H319, H334, H335, H351, H373, H410 |
| Precautionary statements | P260, P264, P270, P271, P272, P273, P280, P284, P302+P352, P304+P340, P304+P341, P305+P351+P338, P308+P313, P312, P314, P320, P330, P363, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0-⋆ |
| Autoignition temperature | 720°C |
| Lethal dose or concentration | LD₅₀ oral rat 4000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2820 mg/kg |
| NIOSH | SY2450000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.11 mg/m3 |
| IDLH (Immediate danger) | 100 mg/m3 |
| Related compounds | |
| Related compounds |
Chloranil
Phosgene Terephthalonitrile Tetrachlorophthalic anhydride |
| Related compounds |
Trichloroterephthalonitrile
Tetrachlorophthalic anhydride Tetrachlorophthalonitrile Terephthalonitrile Chloranil |
| Pharmacology | |
| ATC code | N06AX05 |
