Tetrachlorophthalonitrile: Development, Characteristics, and Prospects
Historical Development
Tetrachlorophthalonitrile has roots stretching back to the mid-twentieth century. Driven by the demand for complex organic molecules in pigment synthesis, chemists began to explore halogenated phthalonitriles. This compound quickly drew notice among manufacturers seeking better precursors for high-performance phthalocyanine pigments. By the 1960s, the paint and plastics industries carried out more work with tetrachlorinated precursors, turning this niche molecule into a backbone for blue and green colorants in industrial coatings. Production steps sharpened over each decade, allowing greater yields and cleaner products. Watching the way raw material sourcing tightened up, especially under rising environmental standards, turned this molecule from a novelty to a candidate for broader commercial use.
Product Overview
The pure material appears as an off-white to yellowish crystalline powder. Producers mainly value it as a stepping stone for phthalocyanine pigments, though other specialty uses continue to emerge. Quality standards tend to focus on the purity of the nitrile and minimal contamination from related byproducts. Most suppliers offer it in industrial-scale drums, using nitrogen packing to prevent decomposition over storage. Chemists in pigment houses often keep it on hand because shifts in phthalocyanine blue market demand can rise quickly, requiring flexible synthesis planning.
Physical & Chemical Properties
Tetrachlorophthalonitrile melts at just under 200°C, which makes it easy to handle compared to many less stable organic intermediates. Its structure, built around a phthalonitrile core with four tightly bonded chlorine atoms, delivers impressive thermal stability. Insoluble in water, the powder dissolves in organic solvents like DMF, DMSO, or chlorinated hydrocarbons. Those chlorine atoms block typical nucleophilic attacks, so it stays inert under normal storage and use. Yet the same features that keep the molecule stable also prompt tough waste-handling rules, since chlorinated aromatics don’t break down easily in the environment.
Technical Specifications & Labeling
Quality control teams test most lots for purity above 98%, usually demanding that trace levels of monochlorinated and unreacted phthalonitrile remain below 0.2%. Labels on drums note both the Chemical Abstracts Service (CAS) number—89-05-4—and hazard information related to inhalation and skin contact. Specifications often reference melting point, particle size measured in micrometers, and bulk density. Manufacturers print batch numbers and country of origin for tracking, as global regulatory agencies become more vigilant about sourcing for chemicals used in pigments and specialty plastics. Labeling now reflects demands for hazard communication under Globally Harmonized System (GHS) rules.
Preparation Method
A key method for producing tetrachlorophthalonitrile starts with phthalonitrile as the core skeleton. By treating this compound with an excess of chlorine gas in the presence of an iron catalyst, chemists drive chlorination at four positions on the aromatic ring. This process runs in high boiling organic solvents to enable even substitution. To prevent unwanted side-products, careful control over temperature and chlorine flow remains crucial. Continuous reaction technology has begun to replace older batch chlorination, using better controls and lowering environmental risk by reducing the need for excess reagents. After reaction, crystallization and successive washes extract the pure product from unwanted tar and other halogenated impurities.
Chemical Reactions & Modifications
Tetrachlorophthalonitrile performs as a highly effective precursor for metal phthalocyanine synthesis. Reacting it with urea and various metal salts under high temperature forms intensely colored coordination complexes. It can also undergo nucleophilic aromatic substitution at positions adjacent to the nitrile groups, making it valuable for chemists interested in fine-tuning pigment characteristics. The molecule resists many reductive and hydrolytic challenges, which is why downstream processing often relies on strong bases or nucleophiles to build out new functional groups from the tetrachlorinated core. The chemical’s stability also matters in polymer additive research, where migration and decomposition of the additive must be minimized over years of product life.
Synonyms & Product Names
Many catalogs and suppliers know the material as 3,4,5,6-tetrachlorophthalonitrile. Some pigment manufacturers shorten the name to TCPN. Other legacy trade documents sometimes call it tetrachloroisophthalonitrile, though this misnomer has faded as regulatory harmonization grew. For shipping, United Nations numbers and hazard identifiers often stand alongside the customary IUPAC name to avoid confusion between related phthalonitriles and less-chlorinated derivatives.
Safety & Operational Standards
Handling tetrachlorophthalonitrile requires solid adherence to safety protocols. It poses inhalation and skin contact risks; production lines often install local exhaust ventilation and mandate nitrile gloves and chemical goggles during transfer and weighing. Dusts remain a concern—especially when emptying bags or drums—so industrial hygiene focuses on preventing airborne particles. Following accident reports about older pigment plants, regulatory bodies began raising standards on exposure limits. The industry now relies on better training, stronger material safety data sheets (MSDS), and updated leak-management practices. Disposal gets tied up in environmental controls due to chlorine content—most plants direct waste toward licensed incinerators where complete combustion eliminates hazardous byproducts. Regular air and surface monitoring keeps occupational exposure below established limits, and spill response uses absorbents rated for halogenated compounds.
Application Area
Tetrachlorophthalonitrile has carved a spot in the pigment sector, especially as a cornerstone for phthalocyanine blue and green production. Automotive paint formulators want pigments that deliver consistent brightness and weathering resistance over decades of UV exposure, and starting with high-purity tetrachlorinated precursors makes a difference. Expanding beyond colors, specialty plastics use the compound to prepare stable dyes that won’t migrate or fade in tough engineering applications. Printing ink manufacturers also select it when longevity and chemical resistance prove crucial, such as in security inks for currency or documents. In research, laboratory chemists target it for synthesizing new aromatic materials, modeling after the success of metal phthalocyanines as sensors or catalysts.
Research & Development
University teams and pigment giants both study derivatives of tetrachlorophthalonitrile. Interest grows around green chemistry production—developing catalysts that lower waste and cut energy use during chlorination. Material scientists dig into modifying derivatives to tailor shades or boost lightfastness, using computational chemistry to predict new effects before scaling up at pilot plants. In catalysis, labs experiment with tetrachlorinated phthalonitrile complexes, eyeing their ability to coordinate metals in processes for organic synthesis or environmental remediation. Environmental toxicology tests expand almost every year. Results from those studies guide both product designers and regulatory policymakers who weigh the tradeoffs between performance and health impact.
Toxicity Research
Workplace safety managers watch toxicity data closely. Inhaled dust or skin contact can cause irritation, and animal studies point to possible liver and kidney stress after long exposures. The molecule’s persistence in the environment raises flags that parallel concerns seen with other halogenated aromatics. Some studies suggest possible endocrine interactions, prompting further inquiry from both industry and independent labs. Increasing use in pigments means that regulators want ever-clearer data on biomonitoring and environmental fate, spurring expanded research into breakdown rates, pathways, and possible bioaccumulation risks.
Future Prospects
Demand for tetrachlorophthalonitrile will likely stay tied to the world’s appetite for phthalocyanine pigments, but the push for safer, cleaner production looks set to steer investments in new technologies. Environmental and occupational safety concerns put pressure on producers to innovate process chemistry, so greener chlorination techniques and improved waste controls leave room for progress. Advancements in analytical science could help researchers pick apart environmental impacts at the parts-per-billion level and support efforts to develop next-generation pigments with lower ecological footprints. Startups outside pigments—particularly in electronics and medical diagnostics—consider tetrachlorophthalonitrile as a foundation for advanced functional materials, showing the reach of this chemical continues to grow, even as tighter rules and market shifts reshape the field.
The Backbone of High-Performance Pigments
People who spend their days around paints, plastics, and specialty coatings have likely come across the name tetrachlorophthalonitrile. It’s not the kind of substance folks see headlining in fashion or tech magazines, but in the world of synthetic chemistry, its presence carries a lot of weight.
Tetrachlorophthalonitrile plays a key role as a raw ingredient for making strong, stable pigments. One of its main jobs is helping chemists create phthalocyanine pigments. These pigments show up everywhere—from deep green automotive paints to the bluish shades on plastic chairs scattered in parks. Phthalocyanine pigments last through years of sunlight, temperature swings, rain, and even chemical spills.
Chemistry That Stays Put
In my years working with coating specialists, I learned that once someone finds a pigment that sticks to products and refuses to fade or peel, it earns a long-term spot in production lines. Tetrachlorophthalonitrile helps deliver that kind of reliability. By feeding the pigment-making process, it sets the stage for colors that last, even in tough environments.
A lot of industries want products to look sharp from the very first day right through years of use. Whether it’s a tractor exposed to red clay, a playground bench battered by kids, or a highway sign facing endless sun, pigments derived from tetrachlorophthalonitrile often end up in those coatings. This chemistry holds up under heavy weather and repeated cleaning.
Not Just Paints: Wider Chemical Use
Tetrachlorophthalonitrile isn’t just about creating pretty colors. Factory chemists also use it as a starting block in synthesizing other valuable chemical compounds. Some of these spin-offs include pesticides, herbicides, and specialty resins. Its strong molecular structure brings stability to whatever it’s added to—sometimes even acting as an intermediate in plastics that resist harsh chemicals or heat.
I’ve seen chemical suppliers ship drums of tetrachlorophthalonitrile to several different clients—paint factories, electronics manufacturers, and agricultural chemical producers. Each uses it for specific reasons, but the need for stable, predictable chemistry unites them all.
Handling Safety and Environmental Questions
No substance like this should be handled lightly. It isn’t something for hobbyists or home chemists. Safety data makes it clear: tetrachlorophthalonitrile must be stored and used with gloves, ventilation, and close attention. Spills and leaks pose risks, and breathing in dust or fumes isn’t healthy. I’ve watched chemical plant managers devote real energy to keeping workplace exposure in check, training teams, and following strict disposal practices.
Environmental groups and regulators pay attention, too. Keep chemicals like these out of streams, soil, and landfill dumps. Factory waste gets contained and treated to avoid any chance the product contaminates water or air. Some companies work with third-party auditors who dive deep into every usage record and safety checklist.
Building a Safer Future with Responsible Use
Tougher rules and industry watchdogs sometimes spark complaints among manufacturers, but I’d argue they do important work. Responsible chemical use builds trust with customers—no one wants to see headlines about unsafe products. There are always ways to tighten safety protocols. I’ve met chemists who push for plant upgrades or suggest alternative chemicals, reducing the risks that come with old processes. Finding new uses for byproducts or creating “greener” alternative pigments moves the field forward.
Tetrachlorophthalonitrile sits in the background for most people, but for those making products designed to last, it remains a pillar. Understanding where and how it fits into industrial chemistry sheds light on bigger questions of safety, innovation, and accountability.
Respecting Chemical Hazards in the Workplace
I’ve seen all kinds of chemicals pass through busy labs, from mild irritants to true hazards. Tetrachlorophthalonitrile fits closer to the hazardous end. Whether you work in manufacturing, research, or a warehouse, you don’t get a second chance if you treat a chemical like this lightly. Even a seasoned lab worker can get complacent, and that’s when accidents happen.
Let’s get straight to the real risks. Exposure puts skin, eyes, and lungs in the firing line. The dust or fumes can damage the respiratory tract, and a quick splash burns the skin or eyes. Some folks get so focused on “it’s just powder,” but powder gets everywhere if you’re not careful. Rushing, sticky gloves, and improper handling all set traps for trouble. Nobody forgets the day a colleague’s poor handling meant a trip to the ER—one careless moment. That sticks with you.
Personal Protective Equipment Beats False Confidence
Proper gloves, goggles, and well-fitted lab coats stop accidents before they start. I only trust gloves made for organic chemicals, since thin or worn-out ones fail at the seams. No shortcuts. I’ve worked with teams where people grabbed whatever gloves sat on the counter. One look at the Safety Data Sheet will change that habit for good. Chemical splash goggles do a better job than safety glasses when powder and fumes show up. A simple dust mask doesn’t cut it either—I’ve seen approved, tight-fitting respirators protect people in tough environments. The extra minute putting these on saves you from a lifetime of regret.
The Importance of Good Ventilation
Working in cramped places or closed rooms turns any spill into a major event. Fume hoods and well-maintained exhaust systems mean inhalation hazards don’t build up where people breathe. I remember one shop where fans stayed on, even after hours, since the minute you slipped up with a scoop, the air quality went south. Investing in good ventilation keeps everyone on the safe side, and nobody complains after seeing the air sampling data improve.
Storage: More Than Just a Label
Lock up Tetrachlorophthalonitrile far away from food, drinks, or break rooms. Placing it in spill-proof containers and storing it below eye level keeps it from tumbling off high shelves. I’ve worked in labs where a jar rolled off and broke because a new hire put it with solvents on the top rack. Shelves with ledges, proper labeling, and sturdy secondary containment stop minor mistakes from becoming disasters. Keep incompatible chemicals apart, since careless storage can lead to dangerous reactions.
Training Makes a Difference
No substitute exists for decent training. Walking newcomers through emergency procedures, showing them the eyewash and shower, and explaining what happens if things go wrong leaves an impression. Mock drills might sound boring, but they help people react fast. I’ve been lucky enough to work where supervisors enforce training and review it every few months. As a result, people actually read the emergency charts and know which protocol fits which spill or exposure.
Proactive Steps for Cleanup and Disposal
If a spill happens, act fast. Scoop up solids with special tools, not your hands, and seal waste in proper containers. Dumping even a half cup of this stuff into the trash can is a rookie mistake that leads to bigger problems. Send all contaminated waste to a licensed handler. Hand-washing stations, spill kits, and clean uniforms at the exit keep everyone from tracking chemicals home. Once you experience a real chemical accident, you never forget to keep the workspace clean and the disposal area locked down.
Looking Beneath the Surface
If you ask folks outside chemical circles, almost nobody has heard about tetrachlorophthalonitrile. That alone raises questions. When a chemical has a tongue-twisting name, it doesn’t make headlines until something goes wrong. This substance pops up in specialty chemistry, dyes, and advanced pigments. For most people, it stays behind facility walls. But ignoring hazards because of obscurity rarely works out.
What the Research Shows
In my time covering chemical safety, I’ve learned to trust rigorous research over rumors. Government agencies and environmental watchdogs have run tests on tetrachlorophthalonitrile. The findings ring an alarm bell, especially for those who handle or live near manufacturing plants. This chemical belongs to a group notorious for making tough-to-break-down molecules. Once released, it sticks around in soil and water. Chlorinated hydrocarbons like this can travel, building up in places that nobody ever thought to look.
Health-wise, studies point to eye and skin irritation if someone gets exposed. Extended contact brings on more serious risks, including potential lung irritation and organ toxicity. Workers have reported headaches, breathing trouble, and odd rashes linked directly to inhaling dust or vapors. Some animal studies connect long-term exposure to liver and kidney issues. Precise cancer risk remains unclear, but most experts believe regular exposure is a big gamble nobody should take.
Real-World Impact Is Always Local
These facts matter, especially in real neighborhoods. Roughly a decade ago, I spent weeks in a town that faced groundwater contamination from a similar chlorinated compound. Families there couldn’t trust tap water; even the soil beneath swing sets raised questions. Once chemicals like this get loose, everyone worries about their children, pets, and gardens. Cleanup costs spiral, and trust in local industry erodes.
Industry managers often argue that strict protocols make accidents rare, yet people working in production see things differently. Glove use, masks, ventilation—these are basic shields, but lapses happen. Cutting corners to save money or speed up production raises risks for communities and workers alike. Transparency usually arrives only if outside agencies force open the gates after something leaks or someone falls sick.
Steps Toward Safer Solutions
We don’t have to accept this kind of gamble as the cost of modern chemistry. Seeking out safer alternatives won’t always come easy. Manufacturers can redesign processes and choose less persistent chemicals when possible. Governments must push companies to reveal usage and emissions data. Inspection with teeth, not just paperwork, helps keep shortcuts at bay. Advocacy by local residents does pay off—history shows that pressure secures cleaner air and water, even from powerful industries.
Speaking personally, I’ve learned that small voices speaking up make the biggest difference. Anyone living near a chemical plant deserves a say in what flows out of those stacks or drains. Demanding transparency and safer materials goes beyond regulation; it’s about fairness and health. Tetrachlorophthalonitrile sits at the intersection of industry know-how and real family life. Until we put safety ahead of secrecy, the risks are never just theory—they hit home.
Understanding Tetrachlorophthalonitrile
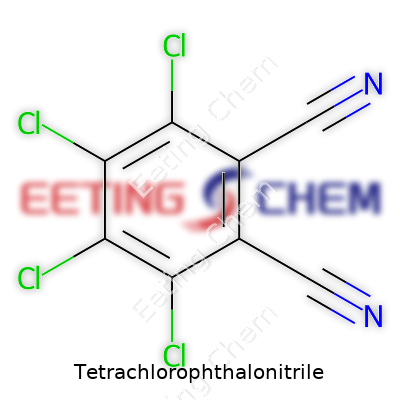
Research and industry often cross paths with complex molecules like tetrachlorophthalonitrile. This compound shows up where synthesis pushes into the realm of pigments, dyes, and specialty electronics. The backbone of this molecule rests on the phthalonitrile framework—two nitrile groups attached to a benzene ring. Four chlorine atoms settle on the ring, which shifts the character of the chemical and gives it unique properties.
The Nuts and Bolts: Chemical Formula and Structure
Tetrachlorophthalonitrile carries the molecular formula C8Cl4N2. Its benzene ring stands as the core. At strategic spots, four chlorine atoms replace four hydrogen atoms. Two adjacent carbon atoms on this ring carry nitrile groups (–C≡N), pulling the full structure into focus. Years ago, working in an industrial lab, I came across phthalonitrile derivatives for organic semiconductors. Each new chlorine atom changed solubility and reactivity. This isn't just trivia; changing those halos reshapes its role in materials science.
If you walk through the structure, visualize this: a six-membered benzene ring, carbons numbered from one through six. Now, imagine chlorine atoms at positions 3, 4, 5, and 6. Nitrile groups claim positions 1 and 2. This lines up in the world of chemical naming as 3,4,5,6-tetrachlorophthalonitrile. The symmetry built into this ring isn’t for show. It guides how the molecule interacts with others, especially during pigment manufacturing or polymer synthesis.
Why Structure Matters
Chemists rarely focus on appearance for its own sake. The location of each chlorine and nitrile group determines how well tetrachlorophthalonitrile dissolves, reacts, or holds up under heat. In pigment production, for example, steric hindrance from the chlorines helps control the crystalline form, which impacts color saturation and lightfastness. This fine tuning can be the difference between a dye that lasts and one that fades right away, like those cheap T-shirts that barely survive a summer’s sun.
Back in the lab, handling tetrachlorophthalonitrile always involved glove boxes and heavy-duty safety glasses. The populated ring means higher reactivity and some real risks. Chlorinated organics like this often raise concerns about toxicity and persistence in the environment. Not everyone talks about this side, but anyone mixing chemicals for a living watches these risks closely. The chemical’s stubborn stability can mean trouble during waste disposal, but industry does best by focusing on responsible use and containment, plus research into alternatives where possible.
Making Better Choices
Tools exist to measure environmental impact and exposure, but balancing commercial need with safety isn’t ever perfect. Industry can invest in closed-loop systems to trap emissions and reuse solvents, cutting exposure all around. For users, clear labeling and hazard training beat paperwork alone. Supporting research into bio-based alternatives or targeted recycling systems could shift reliance away from problematic chemicals like this one. It’s not about bans or panic, but about better stewardship, sharing findings, and looking ahead before problems set in hard.
Tetrachlorophthalonitrile and Why Attention Matters
I’ve worked around harsh chemicals before, and I’ve seen what happens when people get lazy with safe handling. Tetrachlorophthalonitrile—used in dyes, pigments, and resins—poses serious health and environmental risks without proper management. Skin rashes, burned eyes, or even lung problems show up fast with sloppy habits. Secure storage and smart disposal decisions protect not just workers, but families and neighborhoods living nearby.
Storing the Chemical—Common Sense First
Don’t treat Tetrachlorophthalonitrile like a sack of flour. This stuff requires closed containers made from glass or corrosion-resistant metal. Screw-top jars or certified industrial drums stop leaks and keep out unnecessary moisture. My old shop stored similar poisons inside ventilated metal lockers, bolted down off the floor. Keeping temperature and humidity stable near 20°C helped with shelf life and reduced fumes.
I always say: label everything, and make those labels scream danger. “TETRACHLOROPHTHALONITRILE—TOXIC,” all in bold, with skull-and-crossbones warnings or clearly printed hazard pictograms. Emergency responders look for clear signals during accidents or fires. Mark every bottle with purchase date, too—older stock can crystalize or break down, sometimes becoming even nastier.
Separating Hazardous Chemicals
I still remember an incident where a bottle of pesticide tipped into a shelf of solvents—nothing catastrophic happened, but it could have been ugly. Keep Tetrachlorophthalonitrile away from food, acids, strong bases, and direct heat. Never pile on top of oxidizers or reducing agents. Old habits save lives: always wear gloves, goggles, and long sleeves before opening or moving containers, even for a quick check.
Disposal—Shortcuts Poison the Planet
No short route exists for dumping this chemical. Flushing Tetrachlorophthalonitrile down the drain or tossing it in ordinary trash means trouble for drinking water, fish, and people along the waterway. Municipal waste stations and the EPA have strict rules because groundwater contamination doesn’t clean itself up in a week, a month, or often ever. EPA’s hazardous waste listing covers chemicals like this for good reason.
Trust only licensed toxic waste contractors for collection. They usually store it in secure barrels at special landfills or send it to high-temperature incinerators, where only specialized scrubbers trap the fumes and ash. Every drum gets a haul tag, so if something leaks along the journey, someone answers for it. I’ve watched trucks drive out (with GPS records marking every stop) to facilities with gates, badges, and cameras.
Looking Out for People and the Planet
Laws and rules come after piles of sick workers and polluted rivers forced changes. Reading safety data sheets isn't fun, but the first time you see red eyes or hear the neighbor talk about odd smells coming from a drain, the lesson sticks. Encourage crew members and lab workers to take time cleaning spills properly and record incidents, no matter how small. Management should fund training every year—paying for that peace of mind beats lawsuits or shutdown notices every time.
Solutions: Plan, Don’t React
Few people walk into a job knowing all the risks. Starter checklists, extra PPE lockers, and quarterly waste audits catch trouble before it spins out of control. Neighbors appreciate when companies openly talk about safety, and regular emergency drills help everyone sleep easier at night. If anyone spots outdated stickers or barrels out of place, call it out and fix it.
Respect the danger, keep your spaces updated, and use professional waste handlers. That approach works long after the headlines fade away.
| Names | |
| Preferred IUPAC name | 3,4,5,6-Tetrachlorobenzene-1,2-dicarbonitrile |
| Other names |
2,3,4,5-Tetrachlorophthalonitrile
Tetrachloroisophthalonitrile |
| Pronunciation | /ˌtɛtrəˌklɔːr.oʊˌθæloʊˈnaɪtraɪl/ |
| Preferred IUPAC name | 3,4,5,6-tetrachlorobenzene-1,2-dicarbonitrile |
| Other names |
1,2,4,5-Tetrachlorobenzene-3,6-dicarbonitrile
Tetrachlorophthalonitrile 3,6-Dicyano-1,2,4,5-tetrachlorobenzene TCN |
| Pronunciation | /ˌtɛtrəˌklɔːrəˌθælɵˈnaɪtraɪl/ |
| Identifiers | |
| CAS Number | 117-08-8 |
| Beilstein Reference | 613674 |
| ChEBI | CHEBI:38461 |
| ChEMBL | CHEMBL25734 |
| ChemSpider | 164622 |
| DrugBank | DB14006 |
| ECHA InfoCard | 03b0e7ec-40e6-4b62-97e7-f687c7884588 |
| EC Number | 201-817-4 |
| Gmelin Reference | 60360 |
| KEGG | C18545 |
| MeSH | D014180 |
| PubChem CID | 12416 |
| RTECS number | TF1575000 |
| UNII | 2CGO2V93EC |
| UN number | UN3276 |
| CAS Number | 117-08-8 |
| Beilstein Reference | 146194 |
| ChEBI | CHEBI:34643 |
| ChEMBL | CHEMBL2103838 |
| ChemSpider | 20523 |
| DrugBank | DB14069 |
| ECHA InfoCard | 100.028.929 |
| EC Number | 204-126-2 |
| Gmelin Reference | 87200 |
| KEGG | C18622 |
| MeSH | D013756 |
| PubChem CID | 85521 |
| RTECS number | GF9595000 |
| UNII | Q39X2BB888 |
| UN number | UN3347 |
| CompTox Dashboard (EPA) | DTXSID5020627 |
| Properties | |
| Chemical formula | C8Cl4N2 |
| Molar mass | 291.93 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.63 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.94 |
| Vapor pressure | 1 mmHg (at 150°C) |
| Acidity (pKa) | 1.84 |
| Basicity (pKb) | 5.48 |
| Magnetic susceptibility (χ) | -0.00088 |
| Refractive index (nD) | 1.6800 |
| Dipole moment | 1.8 D |
| Chemical formula | C8Cl4N2 |
| Molar mass | 291.85 g/mol |
| Appearance | White to pale yellow crystalline powder |
| Odor | Odorless |
| Density | 1.65 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.7 |
| Vapor pressure | 3.6 x 10^-6 mmHg (25°C) |
| Acidity (pKa) | 1.12 |
| Basicity (pKb) | 6.46 |
| Magnetic susceptibility (χ) | -0.0004 |
| Refractive index (nD) | 1.638 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.7 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 321.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1122 kJ/mol |
| Std molar entropy (S⦵298) | 298.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -660.2 kJ/mol |
| Pharmacology | |
| ATC code | D08AJ06 |
| ATC code | D08AE29 |
| Hazards | |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled. Causes skin irritation. Causes serious eye irritation. Suspected of causing cancer. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H351, H410, H315, H319 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P304+P340, P308+P313, P312, P321, P332+P313, P333+P313, P337+P313, P362+P364, P391, P403+P233, P501 |
| NFPA 704 (fire diamond) | 3-1-0 |
| Flash point | > 204 °C |
| Autoignition temperature | 510 °C |
| Lethal dose or concentration | LD50 oral rat 2540 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5440 mg/kg (oral, rat) |
| NIOSH | CN1400000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Tetrachlorophthalonitrile: Not established |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; causes skin irritation; causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335, H410 |
| Precautionary statements | P261, P264, P270, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0-🌟 |
| Autoignition temperature | 510°C |
| Lethal dose or concentration | LD50 oral rat 11600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat) 4800 mg/kg |
| NIOSH | SN38800 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Tetrachlorophthalonitrile: 0.1 mg/m³ |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Phthalonitrile
Tetrafluorophthalonitrile Tetrachlorophthalic anhydride Tetrachlorophthalimide |
| Related compounds |
Phthalonitrile
Tetrabromophthalonitrile |
