Pentachlorobenzonitrile: Unpacking Its History, Science, and Impact
Tracing the Path: Historical Development
The tale of pentachlorobenzonitrile runs alongside the broader evolution of synthetic organic chemistry. Chemists sought molecules that let them push boundaries in agriculture, pharmaceutical research, and polymer science throughout the twentieth century. Pentachlorobenzonitrile emerged when researchers delved deeper into halogenated aromatics, especially for their power in modifying biological activity or chemical reactivity. As breakthroughs showed up in the field of pesticides and specialty materials in the 1950s and 1960s, this compound gained specific attention. Anyone with a hand in agricultural chemistry has probably crossed paths with its story: a product from the era when innovation often meant adding chlorine atoms to aromatic rings, chasing stability and selectivity. This legacy still shapes its use today.
Going Beyond the Molecule: Product Overview
This compound takes the form of a white or pale crystalline solid, often stocked in labs and industry for its value as an intermediate rather than a finished good. The real strength of pentachlorobenzonitrile comes from its chemical backbone. Five chlorine atoms cling to a benzene ring, with a nitrile group giving the structure an extra edge. Industrial chemists and formulators choose it for synthesis — not so much for direct use, but because it opens doors to a range of new chemicals, especially for agricultural and polymer markets. Its handle for further transformation gives it a pivotal spot in the manufacturing chain, especially where precision and predictable reactivity matter most.
Up Close: Physical and Chemical Properties
You can recognize pentachlorobenzonitrile by its sharp melting point, generally near 185-190°C, and its well-defined crystalline appearance. No odor signals its presence, but anyone who's worked extensively in chemical labs knows that doesn't always mean safety. Solubility tilts toward organic solvents — it dissolves in acetone and dichloromethane, resisting breakdown in water. These traits speak volumes about storage, use, and safety needs. Chemically stable under normal handling, the aromatic ring plus nitrile combination gives it unique reactivity that specialists value for downstream chemistry. Stubborn against light and moderate heat, this compound still gives in to strong acids or bases, opening up possibilities for those who want to tweak its structure.
Laying Down the Details: Technical Specifications & Labeling
In trade and research, pentachlorobenzonitrile gets measured by purity and particle size. Producers vouch for a minimum 98% purity in most typical batches, labeling drums and jars with batch numbers, CAS registry info, and flags for any residual solvents or by-products. Tightly sealed containers sporting hazard warnings and GHS-compliant pictograms line warehouse shelves. Each shipment clips on a safety data sheet that spells out hazards, storage recommendations, and handling protocols. Regular third-party checks and company lab records back up these metrics, making sure the product stays consistent and traceable — a must, whether you’re working in a pilot plant or just tuning up a research reaction.
The Nuts and Bolts: Preparation Method
Synthesizing pentachlorobenzonitrile leans on a sequence well-known in aromatic chemistry. Typically, the process begins with benzonitrile, taking it through an exhaustive chlorination using agents such as chlorine gas in the presence of a catalyst. I've seen crews work these reactions in jacketed glass reactors, monitoring temperature and chlorine delivery to keep the exotherm under control. Industrial players scale up using similar principles: steady dosing, strong exhaust ventilation, and rigorous temperature checks. Waste management and neutralization equipment operate in tandem — halogenated by-products require strict containment for environmental and regulatory reasons. Synthesizing this molecule may sound straightforward on paper, but every batch pushes a team to balance yield, purity, and safety at every stage.
Reacting and Tweaking: Chemical Reactions & Modifications
Despite its stable core, pentachlorobenzonitrile doesn’t just sit on shelves. Chemists chart out a map of potential modifications, taking advantage of the more reactive points around the periphery. The nitrile group can morph into carboxylic acids or amides with the right hydrolysis conditions, and the chlorines open the door to nucleophilic aromatic substitution. In a practical sense, this means you can install all sorts of functionality — introducing amines, thiols, or alkoxy groups — turning one molecule into dozens of new candidates for crop science, dyes, or polymers. Companies regularly tinker to tune biological activity or make intermediates easier to handle downstream. In the world of synthetic chemistry, pentachlorobenzonitrile represents a toolbox in a single package.
A List of Names: Synonyms & Product Names
Step into any chemical catalog and you’ll see a line-up of names for pentachlorobenzonitrile. Besides its core title, suppliers use terms like 2,3,4,5,6-pentachlorobenzonitrile, or abbreviate it as PCBn. Some trade literature references it under codes assigned by particular manufacturers. The wide range of synonyms isn't just a quirk — it reflects the international flow of chemicals and the way research languages have shifted over decades. Proper naming, upfront on containers and documents, saves confusion and prevents accidental mix-ups during ordering or storage, which matters for safety as much as productivity.
On the Floor: Safety & Operational Standards
Anyone who's worked with heavy halogenated organics knows to take safety seriously. Pentachlorobenzonitrile falls into the category that triggers gloves, goggles, and controlled ventilation. Industry protocols spell out air monitoring because inhalation and skin contact can’t be ignored. Splashes or spills mean dash for the eyewash or drench shower — not just for comfort, but to prevent real harm. Facilities stick to closed handling systems as much as possible, reducing the risk of accidental emission. Regular staff training, safety audits, and up-to-date hazard communication form the backbone of every operation. Disposal never ends up as an afterthought: the chlorinated structure resists easy degradation, so proper incineration or certified chemical disposal contractors step in to close the loop.
Real-World Wind: Application Area
Fields as different as agriculture, dye chemistry, and advanced materials lean on pentachlorobenzonitrile for its flexibility. In crop science, it's a building block for certain herbicides — not an active ingredient, but a critical stage in the assembly line. I've seen development chemists work out routes to fluorinated and aminated derivatives, each unlocking a fresh set of biological profiles. The dye industry picks up on the molecule’s halogen load to develop robust, sunfast pigments. Materials science researchers value the molecule for specialty polymers that can withstand tough conditions — a trait born from its highly substituted aromatic ring. The volume of applications tells the story: pentachlorobenzonitrile slots into dozens of products, always upstream from the main event.
Pushing the Boundaries: Research & Development
The lab work doesn’t stop once a molecule hits the market. Teams at universities and advanced R&D outfits keep finding new angles for pentachlorobenzonitrile. Projects pop up every year — using it as a scaffold for new herbicide leads, testing it as a precursor for next-generation materials, even mapping out greener production routes. The challenge often centers on making synthetic pathways more sustainable, using less hazardous chlorinating agents, or reducing chemical waste streams. Any chemist who’s tried to replace a legacy process with a modern one knows this is no easy fix. Still, the breadth of ongoing patent activity and published research points to plenty of room for discovery. Every new direction can turn into the next breakthrough product line, reshaping how industry approaches both safety and performance.
Health and Environment: Toxicity Research
People pay more attention now than ever to health effects from industrial chemicals. Animal studies have flagged potential issues with pentachlorobenzonitrile, particularly if exposure is chronic or bioaccumulation takes root. Regulatory reviews often call for careful tracking of residues and breakdown products. Informed by this, manufacturers build in rigorous exposure controls, and regulatory agencies pin down strict handling, transportation, and disposal requirements. Environmental groups and watchdog organizations prod for deeper study — something justified by the persistent nature of halogenated aromatics in soil and water. For workers and end-users, clear labeling, protective equipment, and respect for exposure limits remain non-negotiable.
Ahead of the Curve: Future Prospects
The future for pentachlorobenzonitrile ties into ongoing trends across chemical manufacturing. Industry adopts tighter regulatory controls and pushes toward greener, safer synthesis methods. Companies race to wring more value out of fewer hazardous feedstocks, so demand for efficient, selective intermediates remains strong, even as environmental restrictions raise the bar. I see a steady shift toward better waste management, closed-loop production cycles, and the search for alternative reagents. Research teams experiment with catalytic methods that sidestep harsh chlorination steps or deliver the same downstream products with less environmental baggage. Consumer demand for safer, cleaner products pushes innovation. Pentachlorobenzonitrile sits in that pivotal spot — still relevant for what it makes possible, but evolving as expectations shift and sustainable chemistry rises to the forefront.
The Hidden Side of Industrial Chemistry
Pentachlorobenzonitrile doesn’t grab headlines. Walk through most chemical plants, and you probably wouldn’t even see the stuff. Still, its story ties into how food makes it from seed to shelf, why river beds spark worry, and how decisions about simple molecules echo through the environment. I’ve looked up its main uses and talked with crop specialists over the years, so I know this isn’t just another footnote from a dusty chemical registry.
Why Do Companies Use It?
Pentachlorobenzonitrile pops up mostly in the pesticide business. Farmers rely on it while growing cotton and rice because it acts as a building block for other chemicals. The real heavyweight in the list is quintozene, a fungicide that tackles soil-borne fungus. Cotton fields, in particular, face threats like pink rot and other nasty fungal diseases. Without reliable ways to keep those fungi down, yields take a hit, and so does the farmer's paycheck. The companies that produce plant-protection chemicals saw pentachlorobenzonitrile’s potential, turning to it as a go-to intermediate.
I once spoke with a rice grower who struggled with seedling blight. After he started using a fungicide that traces part of its chemistry to pentachlorobenzonitrile, his losses dropped noticeably. He didn’t care about the molecule itself, just that the problem in the field got handled.
Lingering Risks
Working with pentachlorobenzonitrile isn’t simple. Add chlorine to any chemical in five places, and you have a persistent molecule. Tracking its runoff troubles environmental groups near manufacturing sites, especially in China and India where big plants focus on scaling production. Once it reaches water or the soil, it can outlast seasons, tainting crops, fish, and even human health. There’s evidence it can cause skin irritation and other health problems in people exposed directly or over time, prompting governments to monitor its use and production closely.
A few years back, I attended a meeting where researchers discussed chlorinated organic chemicals in drinking water. They warned that unchecked dumping and poor waste control could drive up contamination, which isn’t just bad news for wildlife. The story shifts fast from crop success to health alerts. So, regulations keep circling this compound.
Pushing for Better Solutions
I believe industries making or using pentachlorobenzonitrile ought to lead in handling their waste. The technology to cut emissions already exists. Filters, waste treatment systems, and leak detection tools all work when companies use them. Farmers often trust product suppliers, assuming what gets delivered won’t mess with their land for decades. Governments track chemical residues in food and water, set legal limits, and sometimes ban the most dangerous uses if cleanup proves impossible.
Giving farmers new, safer options matters. Research programs work on developing modern fungicides and pest control agents that break down faster and cause less harm. Big ag businesses can champion change by investing not just in chemistry but also in training and outreach for the folks who use these products every day.
The Road Forward
Pentachlorobenzonitrile sounds obscure, but its track record shows that choices about raw materials matter far beyond the lab. Keeping food safe, water clean, and communities healthy doesn’t start or stop with a single molecule. My experience reminds me that behind each chemical hides a web of jobs, risks, and responsibilities. If you want progress, keep demanding transparency, push for safer practices, and treat every compound as part of the bigger story we all share.
Getting Real About the Risks
Pentachlorobenzonitrile carries a reputation that should never be ignored. It’s not just jargon from a chemistry book—this stuff brings real danger into any space where it’s handled. The chemical draws concern mostly for its toxicity. Breathing in its dust, spilling it on your skin, or even being careless when cleaning up after use can put you in the emergency room with respiratory or nervous system trouble. Reacting with everyday moisture, Pentachlorobenzonitrile can pump out toxic fumes. I remember the strong warning labels that cover every container—those skull-and-crossbones pictures made me stand up straighter every time I took inventory.
What surprised me most was learning that there’s no room for shortcuts when working with Pentachlorobenzonitrile. A single moment of laziness or impatience can have lifelong consequences. Nobody walks away with “just a little” exposure. The safety sheet explicitly lists solid evidence: animal studies confirm it’s seriously toxic, stubbornly persistent in soil and water, and a persistent threat to aquatic life. For the sake of your lungs, liver, and skin—not to mention the environment—a cavalier attitude spells trouble.
Clear, No-Nonsense Precautions
Protecting yourself around Pentachlorobenzonitrile starts with personal gear. I’ve worn goggles that hug my eyes, gloves made for chemical resistance, and disposable overalls, skipping none of it even for a quick clean-up. The air itself needs constant respect: chemical fume hoods or ventilators aren’t “nice-to-haves.” Fresh air is a must, and opening a window won’t cut it. Every workplace I’ve been in required certified respirators, not paper masks.
Touches and spills create the biggest headaches. Skin contact causes both short-term and long-lasting damage, so I’ve seen even seasoned technicians double-checking their gloves for leaks and planning clean-up steps before ever cracking open a container. If liquid splashes onto clothing, it goes straight into a disposal bag—not the washing machine. I’ve witnessed the sheer speed at which safety washing stations get used, and nobody lingers after hearing the training stories of missed spots turning into serious rashes or burns.
What About the Waste?
Disposal gives a whole new headache. Dumping this chemical down the sink or tossing contaminated gear into the regular trash turns an in-house problem into a statewide disaster. Pentachlorobenzonitrile doesn’t break down easily; it sticks around, and its toxicity keeps doing damage. Everyone I know who handles waste coordination stresses the value in labeling everything, locking up storage barrels, and handing hazardous waste off to licensed contractors. If I ever ran out of proper containers, I stopped everything until new supply arrived. Trust evaporates quickly when someone tries to sneak by these steps. State regulators fine harshly and neighbors sometimes suffer—nobody wants to be that person in the headlines.
Solutions that Make a Difference
Training ends up mattering most. Every solid routine I’ve seen in a lab or warehouse starts with drills and actual stories, not just paperwork and theory. People stay in the habit of checking their safety gear, double-bagging everything, and updating checklists. I’ve learned the hard way that people zone out with routine work—rotating tasks and quizzing teammates can save real lives. Regular audits beat last-minute fixes every time. Sometimes management hesitates to pay for all the gear and disposal, but skipping those costs always costs far more in the end.
From my experience, fighting complacency through honest warnings, real supplies, and practical knowledge builds respect for Pentachlorobenzonitrile. Safety doesn’t sell itself, but hands-on awareness does. With chemicals like this, cutting corners only leaves scars.
Pentachlorobenzonitrile: What’s in a Name?
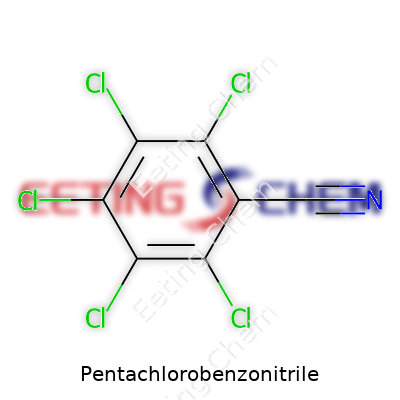
Pentachlorobenzonitrile. The name itself gives away a lot if you break it down. Picture a benzene ring, sturdy as ever, but now it’s armed with five chlorine atoms and a nitrile group. In terms of chemical shorthand, the formula chalks up as C7Cl5N. Each element has its weight, both in the lab and the real world, and this compound sits right at the intersection of chemistry and environmental caution. The formula signals not just a molecular arrangement but a set of real-world concerns, from health risk to industry impact.
Where This Compound Shows Up
Pentachlorobenzonitrile isn’t just a chemistry class puzzle. Its biggest claim to fame comes from the pesticide industry, where it pops up as a byproduct in the making of quintozene (PCNB), a well-known fungicide. I’ve come across its mention in discussions on regulatory safety, especially in agricultural circles. Farmers trust these solutions to keep crops fungal-free, but byproducts like pentachlorobenzonitrile often sneak into the mix. The Environmental Protection Agency and other safety watchdogs keep a close eye on it, and not just for curiosity’s sake.
Health and Environmental Concerns
This isn’t the sort of compound that disappears after use. After applications, runoff or improper disposal can introduce pentachlorobenzonitrile into water sources or fields. Studies have linked this compound to both aquatic and soil toxicity. I learned early on that it can accumulate in the environment, hitting fish and invertebrates hard. Chronic exposure also raises red flags—chlorinated aromatic compounds often stick around and don’t break down easily. The World Health Organization notes potential risks tied to longer-term, low-level exposure, which should grab anyone’s attention who cares about drinking water or food safety.
Why the Chemical Structure Shapes Policy
The formula itself explains a lot. Five chlorines make the molecule heavier and harder for microbes to degrade. This stability is a double-edged sword; while it boosts fungicidal power, it also lingers in places it shouldn’t. Regulatory bodies across North America and Europe have responded by tightening rules about residues in food and soil. Food safety auditors comb through field data, looking for traces. I’ve seen cases where produce shipments got held at border crossings because of elevated chlorinated byproduct counts. So, the structure isn’t just a chemistry footnote—it shapes what goes on supermarket shelves and, by extension, into our bodies.
What Solutions Stand Out?
Byproducts like pentachlorobenzonitrile challenge chemists and industry leaders alike. Better purification and production processes, such as closed-loop systems or more selective catalysts, show promise for cutting down amounts created during fungicide manufacturing. Switching to less persistent alternative fungicides also picks up steam wherever regulations warrant it. On the farmer’s end, using protective equipment and observing longer pre-harvest intervals helps keep residues off crops. I’ve talked with agricultural extension workers who spend whole seasons training growers about best practices for application and disposal.
Looking Ahead
Knowing the formula C7Cl5N isn’t just for passing tests. It’s about understanding the wider story—how a single compound echoes through supply chains, fields, watersheds, and kitchens. Continued research, shared knowledge, and careful policy keep the risks in check, all rooted in something as simple as counting carbon, chlorine, and nitrogen atoms the right way.
Understanding the Risks
Pentachlorobenzonitrile is not just some chemical for lab shelves. It’s a compound with a hefty list of safety challenges. Anyone who’s worked around it knows the sharp, biting smell. Touching or breathing it in makes for a terrible workday—and long-term exposure comes with real health hazards. Skin burns, eye irritation, breathing trouble—the effects can hang around much longer than you'd expect. Reports from the CDC and EPA have documented its toxic effects on both people and the environment. It doesn't break down easily outdoors, sticking around in soil and water, so mistakes echo for years.
Choosing the Right Container
Plastic jugs and old paint cans belong nowhere near pentachlorobenzonitrile. Only containers with solid chemical resistance, like certain steel drums or high-density polyethylene with secure seals, stand up to the job. I’ve seen outdated barrels crack and leach, turning a simple storage error into hours of crisis management. Tight-fitting lids stop leaks and keep fumes locked down. Rust, loose caps, or worn threads have no place here. Any technician worth their salt inspects storage gear before use and scrapes off leftovers or residue right away. One whiff in a cramped supply closet reminds you why.
Safe Storage Settings
Leaving any chemical in direct sunlight or near heat turns it unpredictable. Stacking containers next to hot machines or on windowsills risks chemical breakdown or even ignition. On-site, I’ve always looked for storage spots away from workflow areas. Dedicated, clearly marked rooms with strong ventilation suck away fumes and keep air clean. Every emergency drill I’ve run stressed these points: don’t store near acids or oxidizers, don’t overfill, and never ever store next to break rooms or lunch tables. The rules sound simple, but short-cuts lead to spills and injuries fast.
Environmental Controls
Messes don’t stay local. Spilled pentachlorobenzonitrile soaks into warehouse floors, drains into local water, and can contaminate entire batches of raw materials. Drain covers, proper secondary spill containment, and chemical-resistant flooring Catching leaks fast keeps the outside world safe, too. One cleanup in a rural storage site with no containment turned into a groundwater headache for years after. Smarter planning—like installing sumps and lining storage zones—keeps tragedies out of the news and off your conscience.
Training and Documentation
Every person handling hazardous chemicals should know exact risks and the basics of clean-up. Proper training means the difference between a quick, safe reaction and a panic that causes more harm. I remember a rookie technician who skipped refresher training and ended up with a splash injury; that story made its rounds, reminding us that regular drills aren’t just paperwork. Up-to-date safety data sheets (SDS) hang right near storage, and access must stay clear for emergency teams. Laws in the US require this by OSHA’s Hazard Communication Standard, and ignoring it is just asking for trouble during inspections or real emergencies.
Looking Ahead
No one who values safety or the planet stores pentachlorobenzonitrile without paying attention to every step. Modern storage tech like continuous air monitors, digital inventory, and automated alerts reduce risks. Suppliers who stick by their safety guarantees and provide compliant packaging spare you guesswork and regret. Chemical stewardship means treating each container like it’s a loaded weapon—care learned the hard way keeps everyone out of the ER and the headlines.
Why Pentachlorobenzonitrile Matters
Pentachlorobenzonitrile doesn't pop up in everyday conversation, but it holds weight in some industrial and agricultural circles. Workers in chemical plants and people near manufacturing sites face higher odds of crossing paths with this chemical. It isn’t just a harmless bystander — it can sneak into water, air, and soil. Its persistence can make it a quiet, lingering threat far from its source. I’ve spoken with factory workers who have dealt with chemicals like this, and their worries stick with me. Most of them carry a strong sense of unease whenever toxic names get tossed around on the factory floor.
What Citizens and Workers Risk
Pentachlorobenzonitrile exposure affects the body in ways nobody wants to deal with. It can irritate the skin, making people break out in rashes or feel itchy long after contact. If dusts or vapors reach the eyes or get inhaled, the results aren’t much better. Headache, sore throat, trouble breathing — these symptoms show up if a person keeps getting exposed. People who handle chemicals as part of their job usually stay alert to these warning signs, but in places where rules get ignored, those signs don’t always get respected. Chronic exposure carries greater risks, raising concerns for liver and kidney harm. Scientific journals and public health reports repeatedly link similar chlorinated chemicals to long-term organ stress, so it’s wise not to brush this off.
Long-Term Worries
Prolonged exposure can do more than trigger short-term irritation. Some studies, including animal research, suggest pentachlorobenzonitrile can play a role in more serious health problems with repeated contact. The liver and kidneys have to process and filter out these toxins. Over time, damage piles up, causing pain, fatigue, and sometimes pushing people toward chronic conditions. Some chemicals in this category have been connected to higher cancer risks and immune system disruption. I’ve lost family friends to cancers linked with old industrial exposures, and stories like theirs reinforce the need to sound the alarm about anything with these properties.
What Can Be Done Right Now
The question isn’t just how dangerous pentachlorobenzonitrile might be, but what steps make a dent. Strong air ventilation matters. So does proper protective equipment, including gloves and gloves. I’ve seen safety corners in plants where nobody skips their gear. That makes a difference, as anyone who’s watched a coworker lose time from work due to a preventable chemical burn can attest. Clear handling and disposal instructions on-site spare whole communities from contaminated water or soil. Periodic health checks for workers, along with open communication about symptoms, helps catch trouble early. Cutting-edge filtration systems add another layer of defense. At home, raising local awareness matters — people have a right to know what’s in their air and water if plants operate nearby.
Common Ground for Change
Addressing pentachlorobenzonitrile risk doesn’t require reinventing the wheel. It asks for care, respect for working people, and reliable oversight. I’ve seen strong public health policy make industrial towns safer. It’s possible to keep hazardous exposure at a minimum if leadership, business, and labor groups pull together. Honest talk, education, and action can tip the balance toward safer lives — in plants, neighborhoods, and every spot where chemicals could touch a family’s health.
| Names | |
| Preferred IUPAC name | 1,2,3,4,5-Pentachlorobenzene-1-carbonitrile |
| Other names |
PCBN
Quintozene nitrile PCBN nitrile |
| Pronunciation | /ˌpɛn.təˌklaɪ.roʊˈbɛn.zəˌnaɪ.trɪl/ |
| Preferred IUPAC name | 2,3,4,5,6-Pentachlorobenzonitrile |
| Other names |
Quintozene nitrile
PCBN Pentachlorobenzene carbonitrile PCBN nitrile |
| Pronunciation | /ˌpɛntəˌklɔːrəˈbɛnzoʊnaɪtraɪl/ |
| Identifiers | |
| CAS Number | 82-06-0 |
| Beilstein Reference | 1461822 |
| ChEBI | CHEBI:34918 |
| ChEMBL | CHEMBL62971 |
| ChemSpider | 11772 |
| DrugBank | DB13374 |
| ECHA InfoCard | 100.032.408 |
| EC Number | 206-196-7 |
| Gmelin Reference | 82758 |
| KEGG | C14633 |
| MeSH | D010415 |
| PubChem CID | 8566 |
| RTECS number | GG1400000 |
| UNII | B7B70H46Y2 |
| UN number | UN3077 |
| CAS Number | 82-06-0 |
| 3D model (JSmol) | `4-n15sQnb6JlzqWGSxdrC1fcI9m9rEwjB7FBpjlzb1NbSmTFdcGZK02DqYGZX3Sdk3IIQsmUWkN6n9W+ASV670o1OQRI5zPytUosvJJpGksZRzv1naTSz7yoRhewUsfPQOoZhoL4UptTR4wP8pM9afTmA1cf975AM/` |
| Beilstein Reference | 1208025 |
| ChEBI | CHEBI:34443 |
| ChEMBL | CHEMBL38873 |
| ChemSpider | 15379 |
| DrugBank | DB13570 |
| ECHA InfoCard | 03e1c3ca-da5a-43b1-912b-b17382215f1d |
| EC Number | 206-325-6 |
| Gmelin Reference | 52900 |
| KEGG | C14449 |
| MeSH | D010393 |
| PubChem CID | 8596 |
| RTECS number | GE7400000 |
| UNII | MWS8X04K2C |
| UN number | UN3153 |
| CompTox Dashboard (EPA) | DTXSID3021002 |
| Properties | |
| Chemical formula | C7Cl5N |
| Molar mass | 290.34 g/mol |
| Appearance | white crystalline powder |
| Odor | Odorless |
| Density | 1.68 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.0 |
| Vapor pressure | 8.2 x 10^-5 mmHg (25°C) |
| Acidity (pKa) | 1.36 |
| Basicity (pKb) | 1.56 |
| Magnetic susceptibility (χ) | -0.000157 |
| Refractive index (nD) | 1.6500 |
| Viscosity | 1.38 mPa·s (140°C) |
| Dipole moment | 2.95 D |
| Chemical formula | C7Cl5N |
| Molar mass | 290.34 g/mol |
| Appearance | White crystal |
| Odor | Odorless |
| Density | 1.68 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.3 |
| Vapor pressure | 0.00023 mmHg at 25°C |
| Acidity (pKa) | Acidity (pKa): 2.04 |
| Basicity (pKb) | pKb = 10.91 |
| Magnetic susceptibility (χ) | -0.000141 |
| Refractive index (nD) | 1.655 |
| Viscosity | 1.7 mPa·s (20 °C) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 312.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -57.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1072.8 kJ mol⁻¹ |
| Std molar entropy (S⦵298) | 204.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -44.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1623 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N01AX02 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, may cause respiratory irritation, toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS06, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H301, H311, H331, H410 |
| Precautionary statements | P260, P261, P264, P270, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P314, P330, P363, P391, P403+P233, P405, P501 |
| Flash point | Flash point: 210°C |
| Autoignition temperature | 400°C |
| Lethal dose or concentration | LD50 oral rat 3250 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,120 mg/kg (rat, oral) |
| NIOSH | SN15900 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Pentachlorobenzonitrile: Not established |
| REL (Recommended) | 0.1 mg/m3 |
| Main hazards | Toxic if swallowed, causes skin irritation, causes serious eye irritation, may cause respiratory irritation, very toxic to aquatic life. |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. H410: Very toxic to aquatic life with long lasting effects. |
| Precautionary statements | P260, P261, P264, P270, P271, P273, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P311, P312, P321, P330, P332+P313, P337+P313, P363, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0-☢️ |
| Flash point | Flash point: >230°C (closed cup) |
| Autoignition temperature | 540 °C |
| Lethal dose or concentration | LD₅₀ oral rat 983 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1040 mg/kg (rat, oral) |
| NIOSH | 'PB6475000' |
| PEL (Permissible) | PEL: 0.5 mg/m3 |
| REL (Recommended) | 0.4 mg/m3 |
| Related compounds | |
| Related compounds |
Chlorobenzonitrile
Dichlorobenzonitrile Trichlorobenzonitrile Tetrachlorobenzonitrile Pentachlorobenzene |
| Related compounds |
Benzonitrile
Chlorobenzonitriles Pentachlorobenzene Hexachlorobenzene Pentachlorophenol |
