Commentary on 3,4-Dichlorobenzonitrile: Unpacking Its Role and Impact
Historical Development
People have explored the world of chemicals for centuries, and 3,4-Dichlorobenzonitrile sits as a solid example of progress in applied science. The first syntheses date back to the golden age of industrial chemistry, when researchers zoomed in on ways to add specific groups to aromatic rings. By the early twentieth century, the demands of herbicide development and specialty intermediates pushed the scaling and refinement of these preparations. The chemical found itself at the intersection of innovation and practical demand, shaping how future generations would handle both weed control and the design of more complex products. It’s not just a background player. Its emergence left an imprint on how scientists approach chlorinated aromatic compounds and nitriles in general.
Product Overview
In daily conversations, hardly anyone mentions 3,4-Dichlorobenzonitrile, yet in chemical plants and scientific labs, it plays a central role. This compound strikes a balance between stability and reactivity, unlocking routes for both manufacturing and research. Both powder and crystalline forms circulate in the market, making it flexible enough for direct use or further transformation. It finds its way into herbicide blends, fungicidal agents, and intermediates that feed into dyes, medicines, or agricultural chemicals. Workplaces handling this compound bank on its properties, not just for end-products but as a cog in many chemical processes.
Physical & Chemical Properties
3,4-Dichlorobenzonitrile manifests as a white to off-white crystalline solid, with a sharp, almost acrid scent that gives away its chemical roots. It weighs in with a molecular mass of 172.02 g/mol, thanks to its two chlorine atoms and the cyanide group. The melting point hovers around 50 to 54°C, making storage straightforward in most climates. Solubility sways heavily toward organic solvents like acetone, dichloromethane, or chloroform while water barely pulls any of it in. The chlorine groups make the ring more electron-deficient, which affects how the compound behaves with nucleophiles and electrophiles alike.
Technical Specifications & Labeling
Anyone ordering or managing inventories knows documentation matters. Specific lots of 3,4-Dichlorobenzonitrile often carry assay details, impurity content, and moisture limits. Most manufacturers report purities hovering at or above 98%. Packaging requires tough containers with non-reactive linings; exposure to air and moisture could threaten both safety and quality. Every drum, bottle, or bag comes labeled with hazard pictograms, UN numbers, and handling instructions, all geared toward protecting workers and emergency crews. Compliance with GHS (Globally Harmonized System) stands as a hard requirement, showing that the industry takes transparency and risk reduction seriously.
Preparation Method
Industries rely on practical, scalable synthesis. The classic route starts with 3,4-dichlorotoluene, which undergoes catalytic ammoxidation with ammonia and an oxidizing agent under elevated temperature. This approach takes advantage of the methyl group’s reactivity, swapping hydrogen for the skilled addition of a nitrile group, yielding the target molecule. The process spits out less hazardous byproducts than some older techniques, showing people learn from experience and adjust for cleaner processes. Some laboratories still follow alternative pathways such as halogenation followed by Sandmeyer-type reactions, especially when tighter control over side products is crucial.
Chemical Reactions & Modifications
Chemists love tuning molecules. The two chlorine atoms on the aromatic ring open lots of doors for functionalization. Common transformations include nucleophilic aromatic substitution, where groups like amines or alkoxides take the chlorine's place. The nitrile group itself gives options — hydrolysis leads to carboxylic acids, while reduction brings it down to the level of amines. These modifications don’t just serve academic curiosity; downstream industries count on them to create fungicides and pharmaceutical intermediates. Working with these routes, I’ve noticed that careful temperature control can make or break a reaction’s selectivity and yield, proving that expertise isn’t just about knowing a recipe, but about reading subtle cues along the way.
Synonyms & Product Names
The chemical finds multiple names in trade and research catalogues, including Bichlornitrile, Benzonitrile, 3,4-dichloro-, and 3,4-DCBN. International chemical suppliers sometimes assign proprietary designations or trade numbers, and different regions adopt their own conventions. If you’ve dealt with global procurement, you’ll know the importance of double-checking these synonyms to avoid costly delays or mismatches.
Safety & Operational Standards
Workplaces treat 3,4-Dichlorobenzonitrile with respect. It does more than irritate the eyes and skin — inhalation or long exposure can trigger chronic effects. Standard operating procedure means closed systems, local exhaust ventilation, splash-proof clothing, and vigilant emergency measures. Material Safety Data Sheets build resilience into workplace culture, detailing everything from spill response to chronic hazard evidence. In my time handling aromatic nitriles, regular environmental monitoring and personal protective gear have always proven worth the time and resource investment.
Application Area
This molecule earns most of its commercial keep as a herbicide or as an intermediate for more complex agrochemicals. In fields, it disrupts cell division in weeds, offering farmers a precise tool against hard-to-control plants. Further downstream, pharma companies search for scaffolds just like this to experiment with new medicines targeting both human and veterinary diseases. Dye manufacturers also tap its reactivity to build vivid, robust pigments that won’t break down in harsh environments. Each sector brings its own twist on process and quality requirements, proving that one molecule can spark dozens of outcomes.
Research & Development
Academic and industrial labs keep digging for new uses and safer ways to manufacture 3,4-Dichlorobenzonitrile. There’s steady interest in greener catalysts, solvent-free techniques, and biotransformations that cut hazardous byproducts. The ongoing search for new biological targets for herbicides, antifungals, and antimicrobial agents regularly circles back to this chemical or its close relatives. Collaborative work between synthetic chemists, toxicologists, and field biologists often steers these projects, highlighting the connections between benchwork and real-world application.
Toxicity Research
No one working in the sciences shrugs off toxicity. Studies show acute exposure may trigger respiratory distress and skin inflammation. Recent animal studies continue to map organ-specific liabilities, with chronic dosing correlated with hepatic and renal impacts. Environmental scientists watch its runoff because of potential bioaccumulation in soil and aquatic food chains. Regulators in Europe, North America, and Asia have ramped up reporting rules, and pesticide re-evaluations lean heavily on independent risk assessments. While some improvements in formulation have helped cut dangers, researchers and risk assessors keep pushing for less persistent, more biodegradable alternatives.
Future Prospects
As markets shift more toward sustainability and health, 3,4-Dichlorobenzonitrile faces both challenges and new opportunities. Technologists and entrepreneurs actively explore more eco-friendly pathways, renewable starting materials, and targeted formulations that do less collateral damage to non-target species. There’s also growing demand for advanced derivatives in both polymer and specialty chemical areas, driven by trends in electronics, coatings, and next-generation medicines. Close partnerships between industry, regulatory bodies, and academic consortia will likely shape the next wave of developments. Anyone closely following specialty chemicals sees the writing on the wall: adapt to greener chemistry or watch older products get pushed aside by smarter, safer alternatives.
Chemistry’s Utility Player for Herbicide Production
3,4-Dichlorobenzonitrile crops up a lot in talks about agriculture and chemistry. If you've ever worked on a farm or paid attention to the tricky work that goes into growing crops, you start to understand why certain chemicals become household names among professionals. This compound stands out because it forms the backbone for several selective herbicides. Farmers rely on these products to knock back weeds without harming essential plants. Without effective weed control, crops compete with wild plants for resources, which means less food on the table by harvest season. American soybean or cotton growers often lean on herbicides built on compounds like 3,4-Dichlorobenzonitrile to keep stubborn weeds from cutting into yields. Hard data from the USDA shows even a slight uptick in weed growth can chip away at crop production and mess with livelihoods.
A Role in Chemical Manufacturing
This compound plays a support role in chemical synthesis. In my college chemistry classes, professors loved using real-world examples to show how these “building block” chemicals piece together much bigger, more complicated molecules. 3,4-Dichlorobenzonitrile provides the structure chemists need to assemble specialty chemicals, such as dyes or pigments. Most of what ends up in a can of vibrant paint or an industrial dye starts from raw materials like this. Without it, manufacturers would face supply chain headaches and higher production costs.
Pharmaceuticals and Fine Chemicals
While the average person may only think about medications when picking up a prescription, there’s a long journey behind each pill. During my internship at a mid-sized pharmaceutical lab, I learned that many drugs need rare base compounds. 3,4-Dichlorobenzonitrile often acts as a stepping stone during pharmaceutical synthesis. It gets used during intermediate steps as chemists construct antiviral or antifungal agents. Smooth access to these essential chemicals makes it easier to push new ideas from a researcher’s notepad to a shelf at the local pharmacy.
Challenges and Sustainability Concerns
Use of this compound comes with baggage. The environmental impact of herbicides based on 3,4-Dichlorobenzonitrile draws the attention of regulators and eco-conscious groups. Some runoff from treated fields can show up in local water supplies. Reports from environmental agencies set off alarms about contamination that might threaten aquatic life or seep into drinking water. Once, at a civic meeting about farmland, we heard from a local farmer who used multiple chemical products. He said, “You’re always weighing the need for strong weed control against what’s safe for the neighborhood.”
So what would help? Tighter oversight and regular field testing can catch overuse early. Supporting programs that teach farmers about targeted application or promote integrated weed management creates better habits in the field. Big companies have started to invest in greener versions of older chemistry, exploring biodegradable or less toxic alternatives. Research funding aimed at understanding long-term effects, along with incentive programs for safer farming, points toward smarter use. It turns out that the big story with chemicals like 3,4-Dichlorobenzonitrile is less about whether to use them at all and more about how to handle them responsibly—balancing demand, environmental safety, and the continued push for innovation.
Digging Into the Core Structure
3,4-Dichlorobenzonitrile stands out as a classic case of subtle change, big impact. Its chemical formula, C7H3Cl2N, points to a fairly straightforward structure: a benzene ring hosting two chlorine atoms at positions 3 and 4, along with a nitrile group attached to what chemists call the “benzo” framework. For many in agriculture and those handling specialty chemicals, just seeing those numbers triggers a whole chain of events, from regulatory checks to safe handling decisions.
Why Details Matter in Everyday Chemical Work
Through my own background working in labs and talking to folks in crop management, people want more than a formula. They look for signals. Each atom packs significance. Take the pair of chlorine atoms. These aren’t just seasoning for a molecule. They turn a basic benzonitrile into something that offers unique weed control. In fact, 3,4-Dichlorobenzonitrile, known as DCBN or “dichlobenil” in the field, gets blended into certain herbicides. Gardeners, farmers, even city maintenance workers lean on it for root control and keeping certain weeds at bay.
Handling and Health: Not Just Numbers and Letters
Structural formulas might excite a chemist, but safety officers and workers care about what exposure means. DCBN brings a sharp toxic profile alongside its usefulness. Direct skin contact, inhalation, or even long-term exposure can lead to health problems. The CDC and EPA have both flagged it for careful monitoring. Mistakes, like improper storage or casual disposal, lead to risks for both workers and the environment. That formula—C7H3Cl2N—may look tidy, but it demands respect.
The Environmental Conversation
Communities have reason to watch these chemicals. Runoff from treated areas, buildup in water sources, and unintended contact have all been logged as real-world concerns. Researchers track soil persistence and breakdown rates for DCBN. Some studies show residues can linger for months. Watching regulatory developments, you’ll see agencies push for safer application methods and review allowable concentrations, especially near sensitive ecosystems. Urban applications, especially those targeting roots in sewer lines, often spark debate about alternative treatments or eco-friendlier substitutes.
Better Safety, Smarter Use
Tackling dangers with chlorinated benzonitriles means combining training and technology. Regular PPE use, well-maintained storage containers, and clear label instructions go a long way. Some large-scale users have tried switching to precision spraying or even buffer zones to cut down on off-target impacts. Those conversations start at the supplier, but ground-level workers hold the knowledge needed to spot problem areas before they snowball. Fewer accidents show up where front-line workers get reliable training and the means to ask questions about what’s in those unlabeled jugs or tanks.
Building on Established Knowledge
Academic teams have mapped how the molecule breaks down and how it interacts with other substances in soil or water. This kind of research puts real, user-driven data in front of policymakers and field workers. No one wants to walk blindfolded into a job, especially with chemicals that have bite behind them. That’s where community sharing and education play a role: passing along what works and what to avoid with DCBN keeps everyone a bit safer.
Understanding the Risks
I’ve spent more than a decade working with chemicals in research and industry, so few things sharpen your focus like opening a package labeled “toxic.” 3,4-Dichlorobenzonitrile (DCBN) falls into that category. This pale crystalline substance brings real risk: skin burns, irritation to the eyes and nose, and if it goes airborne, the lungs get the worst of it. Just reading the safety data sheet isn’t enough. You find out quickly why strict rules keep incidents to a minimum.
Protecting Yourself Starts with the Basics
Full-length lab coats don’t just keep your clothes clean—they turn a potential skin exposure into a cleanup job instead of a trip to the emergency room. Splash goggles seal tightly so fumes or droplets don’t surprise you in the middle of a pipetting job. I always glove up twice, using sturdy nitrile gloves, swapping the top pair often. Standard latex gloves aren’t tough enough against solvents found in some solutions of this chemical.
DCBN gives off dust and fumes if you open the container roughly. Keep it inside a fume hood, where a steady airflow drags any vapor away from your face. Years ago, I saw a colleague crack open a jar directly on a bench. She blinked for hours and quit early that day. With eye and respiratory irritation, the lesson was painful and quick—always work with this substance under real ventilation.
Safe Storage and Preparation
You can’t drop DCBN anywhere on a shelf. Set it high and away from moisture, acids, or bases. Moisture leaks or broken bottles ruin a cabinet, but a spill eats through labels and mixes with other chemicals, inviting fires or toxic vapor clouds.
Spills remind me of a scare we had once. Someone skipped using a spill tray, and powder ended up everywhere. Absorbing it using inert material—like sand or commercial pads—worked, but only because everyone was already in gloves, masks, and coats. No one tried to sweep or vacuum until we checked the filters and knew our gear could handle it. Emergency showers and eyewash stations nearby aren’t for show—they stop a small incident from becoming a disaster.
Training and Teamwork Matter
People who handle DCBN owe it to themselves and their teams to keep skills fresh. New staff members never handle toxic chemicals alone. We walk through a dry run, explain the risks out loud, and check PPE before even opening the container. Safety drills uncover hidden problems in the lab setup, like blocked exits or empty eyewash bottles.
Every workplace I’ve known invests time in labeling, updated safety sheets, and visible emergency plans. Without that structure, small shortcuts lead to big trouble. Records of inspections, regular PPE checks, and organized waste programs protect not just one person, but the whole building.
Solutions for Safer Workplaces
Strict procedures transform risk into routine. Replacing cracked gloves and worn labels costs pennies compared to the price of hospital visits and lost work. Talking honestly about near-misses builds trust, and clear signage means nobody forgets what’s inside a crowded cabinet.
In my experience, the best defense sits in habits, not just hardware. Consistent training, team accountability, and open communication all add up to fewer mistakes. Rules keep hands and lungs safe, but culture makes those rules stick. With chemicals like 3,4-Dichlorobenzonitrile, that mindset keeps accidents rare and reputations strong.
Understanding 3,4-Dichlorobenzonitrile
3,4-Dichlorobenzonitrile finds its way into many workplaces—mostly where chemical synthesis or agrochemical production happens. Exposure can cause skin, eye, and lung irritation, and anyone dealing with it should understand the chemicals in their care demand more than everyday caution. In some of the labs I’ve walked through, it’s always the small failures in daily routines—like letting a cap go loose, or forgetting to label a secondary container—that cause real trouble down the road. Chemicals with chlorine atoms and a nitrile group can turn nasty, especially if handled with a casual approach.
Storing for Safety
Don’t leave this compound around just anywhere. It won’t blow up like gasoline, but heat and sunlight can cause breakdown or volatility issues. Even a brief exposure to moisture or direct light can make things worse and speed up degradation, causing odors or potential health threats. Store 3,4-Dichlorobenzonitrile in airtight containers made of compatible materials, keeping it away from acids, bases, oxidizing agents, or anything that could react unpredictably. Melting point shifts, odd clumping, or strange colors seem unimportant until you realize these signal breakdown and risk.
Think about temperature—this isn’t sugar or salt. Keep it in a cool place that maintains steady temperatures. Fluctuation can start chemical reactions or condensation, especially in warehouses that bake or freeze with the seasons. I’ve seen rusting lids, off-gassing, and container bulges in out-of-sight storage closets: all strong cues for someone taking safety seriously.
Environmental Protection
I grew up near a stretch of farmland where herbicides and related chemicals got stored poorly, and local streams paid for it. Chemical runoff can hurt soil and water health. Floor drains, cracks, and open shelving make for perfect escapes if a spill happens. The law in many countries asks for chemical-proof flooring and bunding, but the best lesson comes from watching small leaks add up. Store chemicals well off the ground, away from drains, and locked up to stop tampering or accidental use. Labels need to face the aisle and be clear enough for anyone—including emergency responders in a panic—to read easily.
Personal and Facility Security
Unauthorized access sounds like a distant concern until the day someone mistakes toxic dust for ordinary powder, or kids play in an unlocked warehouse. Straightforward rules limit who comes near these compounds, and inventory logs keep everyone honest. Restricted keys and camera coverage help, but watchfulness and a sense of responsibility do more than the best lock. In my experience, simple habits matter: always check lids twice, keep the main log up to date, never ignore a missing label.
Seeking Solutions and Preventing Problems
Better training stands out as the simplest step. I’ve seen dramatic improvements with just two hours of hands-on safety practice every few months. Staff should run mock spills or container failures, which is the best test for both procedures and reflexes. Regular audits find hidden risks, and staff rotation ensures nobody grows complacent or cuts corners.
Technology helps: automated environmental monitoring, digital inventory systems, and reliable signage pay back every dollar over years. None of this replaces common sense and respect for the chemicals themselves. Responsibility means taking every step—big and small—seriously, because experience keeps showing the worst accidents always start with the smallest oversight.
A Closer Look at Everyday Exposure
Plenty of folks may never have heard of 3,4-Dichlorobenzonitrile, but it shows up more often than one might think, especially in places where weed killers get used. This chemical, found in some herbicides, can turn up in fields, parks, and gardens. For decades, I’ve worked with agricultural communities that rely on such products, and I’ve seen concerns about chemical exposure move from casual conversation to urgent necessity.
How This Chemical Impacts the Body
Direct contact with 3,4-Dichlorobenzonitrile can irritate the skin and eyes. Breathing in its dust or fumes may bother the nose or throat, and, in some cases, bring on headaches or dizziness. Folks working with this substance on a regular basis sometimes don’t realize their symptoms stem from the chemical. The U.S. National Institutes of Health points out that prolonged skin contact or inhalation may cause rashes or breathing troubles, especially among people with allergies or asthma. From my own experience reporting on rural health, field workers often downplay mild symptoms, chalking them up to fatigue or dust, not always recognizing low-level chemical exposure as the cause.
Long-Term Exposure—A Hard Truth
Research on long-term effects reveals even more worrying patterns. The European Chemicals Agency identifies 3,4-Dichlorobenzonitrile as a possible carcinogen. Chronic exposure may damage the liver or kidneys over time. People working in manufacturing plants or applying herbicides in fields, who may lack proper protective gear, face these dangers most directly. Once, during an interview with a farmhand from Southern Illinois, I heard a firsthand account of mysterious headaches and rashes linked back to herbicide exposure. After lab testing, the doctors traced the symptoms to this chemical, making the long-term threat feel personal and immediate.
Ripple Effects—Beyond the Individual
This hazard doesn’t end with workers. Communities living near treated fields or manufacturing sites face worries about contaminated air, soil, or water. A study published in “Environmental Science & Technology” traced chemical run-off from farming operations into local streams, raising alarms about water safety. In small towns, parents worry about kids playing outside or tracking dirt indoors. People who eat produce from contaminated fields want reassurance that their food supplies aren’t carrying unwanted extras. The public rarely gets much transparency about what chemicals drift onto their dinner plates.
How to Lower the Risk
Change starts with proper protective equipment. Gloves, masks, and clothes designed for chemical resistance do more than just meet regulations—they save lives. Companies need to replace old habits with regular training on safe handling and emergency response. Dust masks aren’t enough; full respirators should be non-negotiable for workers mixing or spraying these herbicides. Showing up at public meetings, I’ve pushed for stricter local rules around buffer zones. Keeping homes and schools a safe distance from treated land cuts down exposure, especially for kids and the elderly.
It also falls on authorities to require thorough soil and water testing wherever these chemicals get used. Crowdsourcing local health data can fill in gaps left by slow-moving government agencies. On a personal level, washing produce from unknown sources and demanding traceability from food suppliers strengthen community defenses. Tackling 3,4-Dichlorobenzonitrile’s risks won’t happen overnight, but insisting on greater transparency and enforcing smart, enforceable safeguards can take the sting out of its presence in our environment.
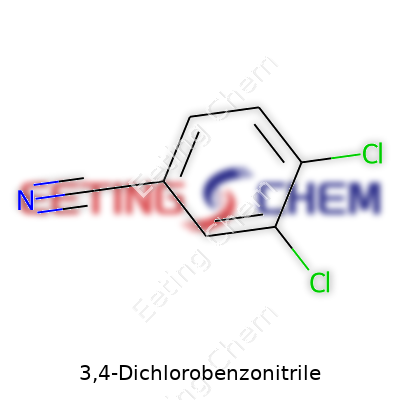
| Names | |
| Preferred IUPAC name | 3,4-dichlorobenzene-1-carbonitrile |
| Other names |
DCBN
Casil Cicedrine Dicol Dichlobenil Herbiblok Prefix Totakil |
| Pronunciation | /ˈθriː,ˈfɔːr daɪˈklɔːr.oʊˈbɛn.zəˌnaɪ.trɪl/ |
| Preferred IUPAC name | 3,4-dichlorobenzonitrile |
| Other names |
Bayer 6035
Casoron Chlorazon Dichlobenil Dichlorbenil |
| Pronunciation | /ˈθriː,ˈfɔːr daɪˌklɔːr.oʊˈbɛn.zəˌnaɪ.trɪl/ |
| Identifiers | |
| CAS Number | 3018-12-0 |
| 3D model (JSmol) | `3D model (JSmol)` string for **3,4-Dichlorobenzonitrile**: ``` CN(C1=CC(=C(C=C1)Cl)Cl) ``` |
| Beilstein Reference | 1364702 |
| ChEBI | CHEBI:132577 |
| ChEMBL | CHEMBL16704 |
| ChemSpider | 13754 |
| DrugBank | DB08703 |
| ECHA InfoCard | 03b50b52-7d22-4f1e-8376-b26253d2267e |
| EC Number | 221-012-9 |
| Gmelin Reference | 137591 |
| KEGG | C14319 |
| MeSH | D003639 |
| PubChem CID | 8508 |
| RTECS number | DY9275000 |
| UNII | CA2VC96XAJ |
| UN number | UN3439 |
| CAS Number | 3018-12-0 |
| 3D model (JSmol) | `3D model (JSmol) string` for **3,4-Dichlorobenzonitrile**: ``` CNc1ccc(Cl)c(Cl)c1 ``` *(This is the SMILES string that can be used in JSmol or related 3D molecular viewers.)* |
| Beilstein Reference | 1261197 |
| ChEBI | CHEBI:34619 |
| ChEMBL | CHEMBL14337 |
| ChemSpider | 14118 |
| DrugBank | DB07932 |
| ECHA InfoCard | 03ccef01-841e-4ca3-8899-c6233a3e1fd2 |
| EC Number | 202-929-4 |
| Gmelin Reference | 775820 |
| KEGG | C14310 |
| MeSH | D003563 |
| PubChem CID | 70844 |
| RTECS number | CZ1925000 |
| UNII | 5XC068V8RK |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C7H3Cl2N |
| Molar mass | 153.01 g/mol |
| Appearance | White to light beige crystalline powder |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 2.9 |
| Vapor pressure | 0.0022 mmHg (25°C) |
| Acidity (pKa) | 1.79 |
| Basicity (pKb) | 1.73 |
| Magnetic susceptibility (χ) | -77.2e-6 cm³/mol |
| Refractive index (nD) | 1.587 |
| Viscosity | 1.603 mPa·s (at 25 °C) |
| Dipole moment | 3.10 D |
| Chemical formula | C7H3Cl2N |
| Molar mass | 204.01 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 2.9 |
| Vapor pressure | 0.0004 mmHg (25°C) |
| Acidity (pKa) | 25.27 |
| Basicity (pKb) | Basicity (pKb): 9.12 |
| Magnetic susceptibility (χ) | -68.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.595 |
| Viscosity | 1.253 cP (25°C) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 189.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -61.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -407.7 kJ/mol |
| Std molar entropy (S⦵298) | 274.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 97.5 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -6064 kJ/mol |
| Pharmacology | |
| ATC code | N06AX15 |
| ATC code | V01XA04 |
| Hazards | |
| Main hazards | Harmful if swallowed, toxic in contact with skin, causes serious eye irritation, may cause respiratory irritation, very toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335, H410 |
| Precautionary statements | P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P337+P313, P362+P364, P403+P233, P405, P501 |
| Flash point | 86 °C |
| Autoignition temperature | 540°C |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): 640 mg/kg (oral, rat) |
| NIOSH | BY5425000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL: 5 mg/m3 |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation, toxic to aquatic life with long lasting effects |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. Toxic to aquatic life with long lasting effects. |
| Precautionary statements | P261, P264, P270, P271, P272, P273, P280, P302+P352, P304+P340, P312, P305+P351+P338, P321, P330, P332+P313, P333+P313, P337+P313, P362+P364, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-1 |
| Flash point | 113 °C |
| Autoignition temperature | 540°C |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 670 mg/kg |
| NIOSH | DU0875000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) for 3,4-Dichlorobenzonitrile is "0.1 mg/m³". |
| Related compounds | |
| Related compounds |
Benzonitrile
2,3-Dichlorobenzonitrile 2,4-Dichlorobenzonitrile 2,6-Dichlorobenzonitrile 3,5-Dichlorobenzonitrile |
| Related compounds |
Benzonitrile
2-Chlorobenzonitrile 3-Chlorobenzonitrile 4-Chlorobenzonitrile 3,5-Dichlorobenzonitrile 2,4-Dichlorobenzonitrile 2,6-Dichlorobenzonitrile 3,4,5-Trichlorobenzonitrile |
