2,6-Dichlorobenzonitrile: A Down-to-Earth Look at a Cornerstone Chemical
Historical Development
Chemists started taking a closer look at 2,6-dichlorobenzonitrile during the mid-20th century, a time when the chemical industry saw rapid growth and pushed boundaries in both basic research and practical applications. The drive to discover new agrochemicals and specialty intermediates led researchers to explore halogenated benzonitriles, and this compound stood out for its unique combination of chlorine atoms and the nitrile group. Early patents focused on weed control, with agricultural scientists noticing its effectiveness for specific applications. The gradual adoption in industry marked a shift from broad-spectrum herbicides to more selective and efficient products, changing the way farmers and manufacturers managed crops and chemical processes alike.
Product Overview
2,6-Dichlorobenzonitrile appears as a white to off-white solid, often taking the form of crystalline powder. Industries lean on it as a herbicide and a building block for other chemicals, thanks to its unique chemical skeleton. Firms interested in crop protection or specialty chemicals often see it arrive in bags or drums ready for use, each shipment clearly labeled with purity and hazard symbols. What makes this chemical so valuable isn’t just its direct effects, but also its role in making more complex compounds down the line, giving both basic chemists and applied researchers plenty of material to work with.
Physical & Chemical Properties
At room temperature, 2,6-dichlorobenzonitrile doesn’t attract much attention: it has a mild chemical smell and turns from a solid to a liquid at around 140 degrees Celsius. It resists breaking down in the presence of light or air, which adds to its attraction as a stable intermediate. Its molecular makeup, C7H3Cl2N, gives it just the right balance of reactivity and stability for both storage and handling. Chlorination at the 2 and 6 positions on the benzene ring confers greater resistance to certain metabolic pathways, which, in practical terms, plays a role in its environmental behavior and persistence in soils.
Technical Specifications & Labeling
Producers of 2,6-dichlorobenzonitrile publish detailed batch specifications, often emphasizing purity (usually above 98%), appearance, melting point, and loss on drying. Packages must state hazard codes associated with toxicity and environmental danger. Responsible vendors include guidance on storage—preferably in cool, dry, and well-ventilated spaces. In handling this chemical, firms must pay attention to trace impurities, which sometimes result from process variations, and they monitor these carefully to meet customer demand for consistent quality and safety standards. No serious buyer ignores the need for chemical data sheets and transport regulations, given both safety rules and potential customs complications.
Preparation Method
Production of 2,6-dichlorobenzonitrile often starts with dichlorobenzene. By introducing a cyanide group via a process called ammoxidation, chemists swap out a hydrogen atom for the nitrile group in the right spot. This method, set at elevated temperatures with the right catalysts, gives the best yield. Some labs tweak the process to cut costs or reduce by-products, using either copper-based catalysts or alternative routes through benzonitrile intermediates. Commercial producers, aiming for scale and low waste, tweak everything from temperature to solvent choice, always weighing efficiency against worker safety and environmental impact. A good friend of mine working in a mid-sized specialty chemicals plant once mentioned how even a one-degree temperature difference could tilt the balance between high yield and expensive clean-up, a daily reminder of the fine line between precision and practicality.
Chemical Reactions & Modifications
With two chlorines and a nitrile in its core, this molecule opens doors to further transformations. Chemists easily substitute the chlorine atoms with nucleophiles in certain reactions to build tailored derivatives, using simple lab setups or complex reactor systems. Under reductive conditions, the nitrile may be converted to an amine, setting the stage for pharmaceutical or agrochemical synthesis. For the classroom or industry bench, it serves as a reliable starting material for halogenated aromatic compounds, and downstream modifications have brought entirely new pesticide classes to market. Its stability toward light and oxidation makes it a favorite for multi-step syntheses, where controlling unwanted side-reactions matters.
Synonyms & Product Names
Walk into any chemical storeroom and people refer to 2,6-dichlorobenzonitrile as DCBN, dichlobenil, or even 2,6-DCBN. Some labels use its systematic name, and pesticide products based on it go by trademarks linked to international registrations. The substance shows up in regulatory documents across continents, and in any global supply chain job, knowing these synonyms helps avoid confusion—especially with dozens of similar-sounding benzonitriles on the market. Chemical catalogues and import records list its CAS number, ensuring clarity between buyers and sellers who might not always share a first language.
Safety & Operational Standards
Dealing with 2,6-dichlorobenzonitrile means suiting up and respecting its hazards. Inhaling the dust or vapors irritates the airways, and in larger doses, exposure can bring headaches or nausea. Skin contact leads to itching or, in some cases, allergic responses. Tight regulations surround its handling, from air filtration at production sites to gloves and goggles for workers. Emergency plans call for careful ventilation and spill control, not just for worker health but also for nearby communities. Storage regulations push for locked and labeled cabinets away from foodstuffs and fire hazards, and disposal always draws attention from environmental bodies. Reading a safety data sheet on a busy shop floor is never just a formality; it’s a practical matter of coming home safe at the end of the day.
Application Area
The most famous use of 2,6-dichlorobenzonitrile lies in weed control. Landscapers, municipal workers, and greenhouse operators rely on formulations that disrupt root development in certain invasive species, keeping horticultural settings in check. Outside of agriculture, manufacturers use it as an intermediate for dyes, pharmaceuticals, and more exotic specialty chemicals. Research groups exploring new materials or synthetic pathways grab this compound as a building block, thanks to its reactive profile and commercial accessibility. Hospitals and clinics rarely see its direct application, but background processes involving 2,6-dichlorobenzonitrile feed the production of several important drugs and agrochemicals, connecting the compound to everyday life in indirect but real ways.
Research & Development
In university labs and industrial R&D wings, scientists keep searching for cleaner and more efficient syntheses and for ways to tweak the core structure of 2,6-dichlorobenzonitrile to produce next-generation herbicides, pharmaceuticals, or advanced materials. Green chemistry efforts push for reduced waste and safer reagents. Data shows that newer catalysts lower energy requirements, leading to less expensive and less polluting manufacture. Researchers in toxicology and environmental science focus on breaking the compound down in the wild, aiming to minimize unwanted accumulation in soils and water. Each avenue keeps the stakes practical: better profit margins, safer working conditions, and a lighter environmental footprint.
Toxicity Research
Years of animal and cell studies show toxic effects at higher doses, with changes in liver enzymes and signs of stress in exposure models. While acute human poisoning is rare, chronic exposure raises questions about subtle shifts in health markers. Regulatory agencies in many countries demand extensive data on both immediate and long-term impacts, especially in agricultural settings where runoff or accidental spills could threaten drinking water or natural habitats. People who apply or package the chemical stay informed on the latest research, pushing for regular medical checks and improved ventilation or isolation protocols. Risk assessment never stops at the lab bench; it follows the chemical all the way through the supply chain.
Future Prospects
Chemists are not finished with 2,6-dichlorobenzonitrile. Ongoing demand in weed management and specialty synthesis anchors its spot in the chemical industry, but tomorrow’s makers will focus on smarter, safer processes and more selective applications. Digital modeling teams pair up with bench chemists to predict new derivatives, and sustainability directors track raw material sourcing to cut down environmental harm. Pressure grows to push for transparency about the compound’s effects in the field and during disposal, and new workshops or regulatory pushes aim to set higher standards. Investors smell opportunity in greener supply chains and novel applications—if scientists can deliver on their promises of both performance and responsibility.
The Role of 2,6-Dichlorobenzonitrile in Modern Agriculture
2,6-Dichlorobenzonitrile doesn’t sound familiar to most folks, but its presence is felt in many fields—literally. This chemical thrives in agriculture as a pre-emergent herbicide, often listed under the trade name dichlobenil. Farmers and groundskeepers use it to keep weeds from sprouting before they can threaten crops, ornamental plants, and public spaces. I’ve spent a good bit of time helping at a community garden, and I’ve seen firsthand the difference a solid weed management plan makes. Unchecked weeds steal the show, draining water and nutrients from the plants that should be feeding us or beautifying a park.
Weed control isn’t only about cleaner rows or prettier gardens. Without it, food prices would swing higher since lower yields mean tighter supply. The U.S. Environmental Protection Agency notes that pre-emergent weed control can safeguard harvests without endless rounds of hand-pulling or repeated tilling that breaks the back. 2,6-Dichlorobenzonitrile works by stopping the growth of a wide spectrum of weeds’ seedlings before they pop through the soil, making the next season lighter on labor for everyone involved.
Beyond the Farm: Other Uses and Risks
This chemical doesn’t stop at agriculture. People put it to use managing weeds in non-crop areas too—think roadside verges, railways, driveways, and even some industrial sites. In my neck of the woods, local maintenance crews use it along fence lines, saving hours each season.
The straightforward impact masks a more complicated reality. Dichlobenil can hang around in soil and make its way into groundwater. Regulatory agencies set strict limits for its use, but improper handling brings risk for wildlife and aquatic life. Over the years, I’ve spoken to families living near treated areas concerned about water quality and how long such chemicals stay active underground. Real risks exist, and ignoring them has led to well-publicized spills and lawsuits. The U.S. Geological Survey has published data showing traces of chemicals like this one in select water sources, which underscores the need for careful stewardship and transparent public reporting.
Tackling the Problem Responsibly
Solutions won’t come from wishing away modern weed problems. What helps is a mix of forward-thinking laws, real-world transparency, and personal responsibility. People applying weedkillers should always read the label, use the right gear, and never go beyond the recommended amount. Local governments monitoring runoff and groundwater can catch leaks before they turn into disasters. I’ve seen community groups press officials for timely data, and their efforts matter. The agricultural industry also has a part to play, leading on research for alternatives—maybe cover crops, or targeted mechanical approaches.
The story of 2,6-Dichlorobenzonitrile responds to a mix of needs: less weeding by hand, steadier crop yields, and tidier public spaces. There’s no denying its role in keeping fields productive and landscapes maintained, but those benefits come with serious responsibilities attached. The more people know, the better choices they can make to balance food production and environmental care.
Understanding 2,6-Dichlorobenzonitrile
Let’s talk straight: 2,6-Dichlorobenzonitrile doesn’t sound familiar to most folks, but this chemical finds its way into plenty of workplaces. Usually tied to agriculture for its weed-killing power, this compound helps farmers and groundskeepers mute invasive plants in fields and along railroads. It’s also used when chemists need ingredients for other manufacturing jobs.
Health Hazards and Exposure
Chemical names aside, people ask the right question: is this stuff hazardous? The answer doesn’t leave much room for doubt. On skin, 2,6-Dichlorobenzonitrile can cause redness, itching, even blistering if there’s enough contact. That’s not a rare story in agriculture, where I remember older hands shaking their heads after spraying without gloves, then itching for days.
Breathing dust or fumes brings headaches, nausea, or even breathing trouble for some. That isn’t just theory; occupational safety agencies recorded cases where exposed workers needed more than a shower and time away from the job. Eyes sting and water in the presence of 2,6-Dichlorobenzonitrile, making protective goggles more than just a nuisance in the field.
Getting it in your mouth or swallowing small amounts leads to even bigger trouble. Stomach pain and vomiting aren’t the worst of it; large enough doses could hit the nervous system. The U.S. Environmental Protection Agency classifies this chemical as hazardous. Animal tests showed harmful effects on organs after repeated contact, which raises real concerns for the people who spend years working near it.
Environmental Concerns
2,6-Dichlorobenzonitrile doesn’t stay where it lands. Rains push leftovers into nearby streams and soil, endangering fish and insect life. Over time, this chemical doesn’t break down quickly, so it builds up—a real worry if you fish or farm downstream. In my own county, local water tests found small traces downstream from a major railroad, and that started a real conversation at the local hardware store.
Wildlife can’t wear gloves or goggles, so every ounce that leaks into their world comes with a price. Fish exposed in research studies showed stunted growth. Insects vanished from test plots—not what you want for healthy soil or backyard gardens.
Limiting the Risk
Good news is, people aren’t powerless. Wearing gloves, long sleeves, goggles, and masks gives a real layer of protection. I’ve watched experienced farmers double-check these basics, knowing how long some rashes last.
Rural communities also share tips about clean-up: plenty of soap and water, not waiting around if you spill on yourself, and never eating or drinking near where chemicals get mixed. Companies with serious safety standards keep labeled drums, so mistakes won’t happen as often. For those managing land, switching to alternative weed-killers or mechanical weed removal trims exposure. Local governments have even started regular soil and water testing to catch problems early.
People can push lawmakers and employers for tighter rules. Many chemical companies provide MSDS (Material Safety Data Sheets) with exact handling steps. Reading and sticking to that advice doesn’t just check a box—it keeps hands steady, eyes clear, and families safer at the end of a hard workday.
A Down-to-Earth Look at This Chemical
2,6-Dichlorobenzonitrile sounds like one of those chemicals you rarely hear about outside specialized industries. In practical terms, it turns up in weed control and the making of other chemicals. From working in labs, I have seen what slack storage does to stability, safety, and even product quality.
Don’t Store It Wherever You Find Room
The powder or crystalline form of 2,6-Dichlorobenzonitrile handles poorly with moisture. Humidity gets in and can cake the product or start chemical changes over time. Standard practice means keeping it cool and dry. Most labs use rooms set between 2°C and 8°C for such sensitive materials, with desiccator cabinets as a backup for powders that pick up water easily.
I’ve seen a handful of careless moves ruin an entire bag. Store this chemical in a tightly sealed, labeled container, far away from the risk of water leaks or damp air. Metal drums or HDPE containers do the job. Place the label so everyone knows what they’re handling. That core habit saves lives and money.
Hazards Get Real If You Skip the Basics
Science teaches respect for harmful substances. Breathing in 2,6-Dichlorobenzonitrile or getting it on skin causes irritation, so nobody wants storage that leads to spills or dust. Workers should use gloves, goggles, and a good lab coat. Chemicals with lingering fumes or fine dust always stay in well-ventilated spots. If it’s handled in bulk, use a chemical fume hood or a spot with local air extraction.
There have been mishaps traced back to using glass bottles with poor seals, leading to moisture sneaking in and turning the material lumpy and far less pure. Someone could skim over inspection and never notice until a process fails. Chemical suppliers recommend tight seals and double-checking for cracks or cross-contamination with other incompatible substances. And never mix this with strong acids, bases, or oxidizers—those can trigger reactions nobody wants to see in a workday.
Choosing the Right Spot in a Storage Room
Don’t treat this material like table salt. Keep it stored below shoulder height to cut down on spills. Lock it up in cabinets made for chemicals. Separate it from potent acids, alkalis, and oxidizing agents on different shelves or, better, different cabinets. The chemical industry learned this lesson the hard way after storeroom fires and accidental reactions that cost people their health and even lives.
Lighting in the storage area matters, too. Some compounds drift when exposed to light, so use opaque or amber bottles if possible. Base your routines on local law and what organizations like OSHA and the European Chemicals Agency recommend. It might feel like extra work, but it keeps disasters off the front page.
Checks, Documentation, and Training
There’s a reason labs track every chemical. An up-to-date inventory and regular checks for leaks, label damage, or container cracks keep operations smooth. Train everyone who handles hazardous compounds and give them all the info from the safety data sheet. In my own experience, people get complacent without reminders—until a close call resets everyone’s priorities.
Every so often, dispose of expired or contaminated 2,6-Dichlorobenzonitrile following hazardous waste rules. Don’t keep more than you actually use, and make safety checks a regular thing, not just a ‘start of the year’ ritual.
Towards Smarter, Safer Storage
Smart storage cuts down health risks and keeps producers, researchers, and the environment far safer. Respect for chemistry, careful habits, and clear rules give teams what they need to manage this material without trouble. In the end, safety is a habit built day after day, not just a list on a wall.
Understanding the Basics
Grasping the structure of a compound like 2,6-Dichlorobenzonitrile starts with its name. The name tells us there's a benzene ring lurking at its core, decked out with two chlorine atoms and a nitrile group. Chemists use shorthand whenever possible, and for this compound, that’s “2,6-DCBN.” It’s a mouthful, but this is the backbone for a range of applications, especially in agriculture and chemistry labs.
The Structure Itself
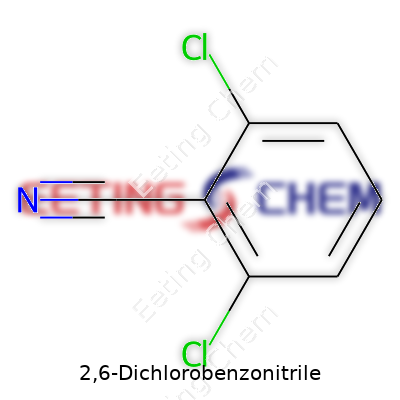
I see the carbon ring — the benzene ring — dotted with two chlorines. One chlorine clings to carbon number two, another to carbon number six. This places them almost across from each other on the ring. The “benzonitrile” part tells you a carbon-nitrogen triple bond sticks off one spot on this ring. To spell it out, the nitrile group replaces a hydrogen on one of the ring’s carbons, making a sharp, linear N≡C bond hanging out of the otherwise hexagonal system.
Chemical formulas don’t always capture the reality of molecules in the lab. For 2,6-DCBN, the formula is C7H3Cl2N. That looks tidy on paper, but pictures from X-ray crystallography give more context: each chlorine is bulky, and the nitrile group points away from the ring, hinting at how the molecule behaves around other chemicals.
Why Structure Matters
I worked in a plant lab for a few summers. Herbicides with 2,6-DCBN stop roots from growing by interfering with cell division. The regular placement of chlorines and the straight-shot nitrile group help this chemical slip into plant tissues, disrupting things at a molecular level. So, these structural details are more than academic—they control how this compound works in the field.
Companies put this knowledge to work. Chemical design depends on hitting the right spots with the right groups. Tweaking those chlorines, or swapping out the nitrile, changes both the efficacy and the environmental fate of these molecules. I’ve seen research looking to design safer versions — ones that break down faster or target specific weeds, not crops.
Concerns and Paths Forward
Controversy trails chemicals like this one. Farmers need weed control, but too much herbicide ends up in rivers, soils, and even our drinking water. The particular structure of 2,6-DCBN lets it linger in the environment. Studies track its impact on non-target species, from frogs to soil microbes. That’s not just my worry; toxicologists and environmental agencies keep flagging persistence as a red flag.
Possible solutions depend on deeper knowledge. Chemists look for ways to tweak the molecule. Adding or moving a chlorine, changing the ring, or modifying the nitrile group itself could help build an alternative that breaks down faster or sticks to its intended target better. I’ve seen promising work that maps how these small changes show up in the real world—sometimes, replacing a single atom shifts the fate of the whole compound.
Looking at the Big Picture
Structure guides function — that’s not just jargon. With 2,6-Dichlorobenzonitrile, small tweaks can have big ripple effects outside the lab. Knowing what each part of the molecule does leads to smarter, safer innovations. If science keeps pushing for transparency and testing, the lessons learned from this compound can help us balance weed control with a healthier planet.
Getting Real About Chemical Exposure
Anyone who's worked with chemicals like 2,6-Dichlorobenzonitrile knows the dangers don’t hide in the label—they live in how you treat the substance day in and day out. This compound, used in some herbicides and specialty products, brings risks that don't always show themselves right away. It can irritate the skin, sting the eyes, or leave you with tightness in your chest after a long shift in the lab or on the factory floor. Safety won’t fall into your lap; it demands action each time you open a drum or pour a powder.
PPE Isn’t a Choice—It’s the Starting Line
Gloves, lab coats, reliable goggles—these aren’t options to consider depending on your mood. They form the baseline for getting close to 2,6-Dichlorobenzonitrile. Make vinyl or nitrile gloves the standard and always double-check that your eyes and face stay covered when measuring or transferring. I once thought a little bit of carelessness wouldn’t matter, but found out quickly that even a quick touch with the skin raised welts. Chemicals rarely give second chances.
Start Thinking About Air
Breathing becomes an issue before you catch a whiff. Work with this compound only in well-ventilated areas to cut out vapor exposure. Fume hoods aren’t decorations—they pull dangerous particles out of the air. In workplaces skimping on airflow, masks with organic vapor cartridges bring down the risk. Workshops and labs owe it to staff to have safety showers, eye wash stations, and real checks on ventilation systems. A clogged hood or flimsy mask fails at the worst possible time.
Clean and Clear Wins Every Time
Keeping benches, containers, and work spaces clean means spills have fewer places to hide. It’s tempting to let a few granules slide, but those stray specks build up into serious exposure over long hours. I’ve seen what happens when shared workspaces turn careless—accidents creep in, and sometimes people pay the price through injuries that could have been dodged with a mop and a dedicated trash bin.
Know Your Spills—Don’t Freeze Up
Spills bring panic, but a prepared worker grabs absorbent pads and tackles the mess without hesitation. Never use water, since that can send dust flying or create runoff. Get the solid substance into sealable containers and treat every spill as if it’s the big one. After years in lab settings, I’ve learned that the fastest way to lose your cool is to realize you don’t know what to do. Regular safety drills create muscle memory, and muscle memory saves you time and injury.
Storage: Organized, Labeled, Secure
Tucked away on chemical shelves, containers without clear labels or with damaged lids end up as accidents waiting for a victim. Store this compound away from incompatible chemicals, always keeping it dry and out of reach of anyone who hasn’t read the safety data sheet from top to bottom. Security doesn’t mean just a locked door; it means a tidy, organized chemical cabinet and a team that never guesses which bottle they’re holding.
Continuous Learning: The Solution That Never Grows Old
Regular training doesn’t just tick boxes—it shapes habits. Workers should have access to the latest safety data, and refresher sessions should challenge them, not bore them. It’s all about building respect for what’s at stake. Safety protocols save lives, but only if people buy in fully. After watching close calls over the years, nothing beats a well-drilled crew that handles 2,6-Dichlorobenzonitrile with attention, respect, and teamwork. That’s how you keep the danger at bay.
| Names | |
| Preferred IUPAC name | 2,6-dichlorobenzonitrile |
| Other names |
2,6-DCBN
2,6-Dichlorobenzene carbonitrile 2,6-Dichlorobenzene cyanide 2,6-Dichlorobenzoic nitrile Dichlobenil |
| Pronunciation | /ˈtuː,sɪks daɪˈklɔːr.oʊˌbɛn.zoʊˈnaɪ.trɪl/ |
| Preferred IUPAC name | 2,6-dichlorobenzonitrile |
| Other names |
2,6-DCBN
2,6-Dichlorobenzene carbonitrile 2,6-Dichlorobenzoic nitrile 2,6-Dichlorobenzene-1-carbonitrile 2,6-Dichloro cyanobenzene |
| Pronunciation | /tuː,sɪks-daɪˌklɔːr.oʊˌbɛn.zoʊˈnaɪ.trəl/ |
| Identifiers | |
| CAS Number | 1194-65-6 |
| 3D model (JSmol) | `-JSmol/jsmol.php?model=C1=CC(=C(C(=C1)Cl)C#N)Cl` |
| Beilstein Reference | 1207936 |
| ChEBI | CHEBI:34598 |
| ChEMBL | CHEMBL27239 |
| ChemSpider | 211059 |
| DrugBank | DB08639 |
| ECHA InfoCard | 03a0df568c-c8d3-47e8-b686-c0644901c992 |
| EC Number | 206-238-9 |
| Gmelin Reference | 86761 |
| KEGG | C14357 |
| MeSH | Dichlobenil |
| PubChem CID | 8449 |
| RTECS number | GE8500000 |
| UNII | J0A2G2JBGG |
| UN number | UN3439 |
| CAS Number | 1194-65-6 |
| Beilstein Reference | 1209240 |
| ChEBI | CHEBI:34681 |
| ChEMBL | CHEMBL42478 |
| ChemSpider | 71434 |
| DrugBank | DB08728 |
| ECHA InfoCard | 19e0eeba-5ccd-4a32-a6e9-2d2041083e95 |
| EC Number | 220-864-4 |
| Gmelin Reference | 136370 |
| KEGG | C19102 |
| MeSH | D003639 |
| PubChem CID | 6856 |
| RTECS number | GO9625000 |
| UNII | YC6YT66PZ3 |
| UN number | UN3432 |
| Properties | |
| Chemical formula | C7H3Cl2N |
| Molar mass | Molar mass: 172.03 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 0.0002 mmHg (25°C) |
| Acidity (pKa) | 1.6 |
| Basicity (pKb) | Product ‘2,6-Dichlorobenzonitrile’ does not have a well-defined pKb value, as it is not a basic compound. |
| Magnetic susceptibility (χ) | -75.1·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.588 |
| Viscosity | 2.09 mPa·s (25°C) |
| Dipole moment | 2.91 D |
| Chemical formula | C7H3Cl2N |
| Molar mass | 204.02 g/mol |
| Appearance | White to off-white crystalline solid |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 0.0021 mmHg (25°C) |
| Acidity (pKa) | 1.8 |
| Basicity (pKb) | pKb: 6.17 |
| Magnetic susceptibility (χ) | -52.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5840 |
| Viscosity | 1.203 mPa·s (25 °C) |
| Dipole moment | 2.47 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 164.6 J mol⁻¹ K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -5.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -573.7 kJ/mol |
| Std molar entropy (S⦵298) | 183.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -46.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –1057 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N06AX15 |
| ATC code | R01AA08 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS06,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 138°C |
| Autoignition temperature | 660°C |
| Lethal dose or concentration | LD50 oral rat 2,288 mg/kg |
| LD50 (median dose) | LD50 (median dose): 640 mg/kg (oral, rat) |
| NIOSH | DY1050000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m3 |
| IDLH (Immediate danger) | 100 mg/m3 |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P362+P364, P403+P233, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 2,2,0,OX |
| Flash point | 138 °C |
| Autoignition temperature | 700°C |
| Lethal dose or concentration | LD50 oral rat 5030 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2,6-Dichlorobenzonitrile: "5,000 mg/kg (oral, rat) |
| NIOSH | SK2800000 |
| PEL (Permissible) | PEL: 10 mg/m3 |
| REL (Recommended) | 0.3 mg/m3 |
| IDLH (Immediate danger) | 20 mg/m3 |
| Related compounds | |
| Related compounds |
2,4-Dichlorobenzonitrile
3,5-Dichlorobenzonitrile 3,4-Dichlorobenzonitrile Benzonitrile 2,6-Dichlorotoluene 2,6-Dichlorobenzamide |
| Related compounds |
2,6-Dichlorotoluene
2,6-Dichlorobenzaldehyde 2,6-Dichloroaniline 2,6-Dichlorobenzoic acid 2-Chlorobenzonitrile 3,5-Dichlorobenzonitrile Benzonitrile |
